| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在稳定表达 GFP-HaloTag7 的 HEK 293 细胞中,HaloPROTAC3 可诱导 GFP-HaloTag7 降解,流式细胞术检测显示,处理 24 小时后,其 DC50 为 19 ± 1 nM,最大降解率为 90 ± 1%。[1]
免疫印迹实验证实,用 500 nM HaloPROTAC3 处理 24 小时后,也能诱导其他胞质 HaloTag7 融合蛋白(包括 HaloTag7-ERK1 和 HaloTag7-MEK1)几乎完全降解。[1] 动力学研究表明,HaloPROTAC3 介导的 GFP-HaloTag7 降解在处理后 4 至 8 小时之间达到 50%。[1] HaloPROTAC3 诱导的降解是可逆的;用 HaloPROTAC3 处理 24 小时后再进行 24 小时洗脱,可观察到 GFP-HaloTag7 水平显著恢复。[1] HaloPROTAC3 的效力和功效显著高于 HyT36(一种疏水标签降解剂),其 DC50 为 19 ± 1 nM(最大降解率 90 ± 1%),而 HyT36 的 DC50 为 134 ± 7 nM(最大降解率 56 ± 1%)。[1] |
|---|---|
| 酶活实验 |
使用荧光偏振实验测定 HaloPROTAC3 与 VHL 的结合亲和力。该实验按照先前报道的 VHL 配体开发方法进行。计算得出 HaloPROTAC3 与 VHL 结合的 IC50 值为 0.54 ± 0.06 μM。[1]
|
| 细胞实验 |
用于降解实验的是稳定表达 GFP-HaloTag7 的 HEK 293 Flp-In 细胞。用不同浓度的 HaloPROTACs(溶解于 DMSO 中)处理细胞 24 小时,最终 DMSO 浓度为 0.1%。处理后,用胰蛋白酶消化细胞,重悬于培养基中,使用 FACSCalibur 流式细胞仪在 FL1 通道上检测细胞内 GFP 荧光。对平均荧光强度进行量化并归一化至 vehicle(0.1% DMSO)对照。绘制剂量反应曲线以确定 DC50 值。[1]
对于免疫印迹实验,用化合物处理稳定表达 HaloTag7 融合蛋白(GFP-HaloTag7、HT7-ERK1 或 HT7-MEK1)的 HEK293T 细胞 24 小时。用含有蛋白酶抑制剂的 RIPA 裂解液裂解细胞。裂解液通过 BCA 法进行浓度归一化,在 10% Bis-Tris 凝胶上进行 SDS-PAGE 分离,转膜至硝酸纤维素膜,用 5% 脱脂牛奶/TBST 封闭,并用特异性一抗(抗 ERK1、抗 MEK-1 或抗 HA)进行孵育。[1] 机制研究涉及用以下物质预处理细胞:1)蛋白酶体抑制剂 epoxomicin(300 nM)预处理 4 小时,然后加入 HaloPROTAC3 再处理 20 小时;2)过量对映体对照 (ent-HaloPROTAC3) 预处理 1 小时,然后加入 HaloPROTAC3 处理 24 小时;3)过量核心 VHL 配体 (VL285) 与 HaloPROTAC3 同时处理 24 小时。通过流式细胞术评估降解情况。[1] 对于洗脱实验,用 HaloPROTAC3 处理 24 小时后,移除培养基,用新鲜培养基洗涤细胞(孵育 30 分钟),然后再次更换新鲜培养基。继续孵育 24 小时后,通过流式细胞术进行分析。[1] |
| 动物实验 |
In Vivo Xenograft Study: BALB/c nude mice bearing HCC-827 xenografts were administered compound 17-1 (20 mg/kg) or vehicle (1% DMSO/60% PEG300/30% normal saline/9% Tween-80) via intraperitoneal injection every two days for 21 days. Tumor volume and body weight were monitored regularly.[2]
Pharmacokinetics and Acute Toxicity Study: ICR mice were administered compound 17-1 (2 mg/kg) intravenously for PK analysis, or a single dose (100 mg/kg) for acute toxicity observation. Formulations for these studies contained mixtures of DMSO, PEG400/PEG300, normal saline, glucose, and Tween-80.[2] |
| 药代性质 (ADME/PK) |
For compound 17-1: Following intravenous administration (2 mg/kg) in mice, the half-life (T1/2) was 11.00 hours, maximum plasma concentration (Cmax) was 7363.33 ng/mL achieved at 0.08 hours (Tmax). The area under the curve (AUC0–∞) was 2939.40 hng/mL, and mean residence time (MRT0–∞) was 3.18 hours.[2]
|
| 毒性/毒理 (Toxicokinetics/TK) |
HaloPROTAC3 showed low toxicity in cell-based assays, as indicated in supplementary figure 3 (not provided in the main text). [1]
HaloPROTAC3 did not affect the stability of HIF (an endogenous target of VHL), as indicated in supplementary figure 4 (not provided in the main text). [1] |
| 参考文献 |
|
| 其他信息 |
HaloPROTACs are a novel class of PROTACs (Proteolysis-Targeting Chimeras) designed to degrade HaloTag7 fusion proteins. They consist of two parts: a chloroalkane moiety that covalently binds to HaloTag7, and a small molecule ligand (derived from (S,R,S)-AHPC) that recruits the VHL E3 ubiquitin ligase. This brings the target protein (fused to HaloTag7) into proximity with the ubiquitination machinery, leading to its polyubiquitination and subsequent degradation by the proteasome. [1]
HaloPROTAC3 represents one of the most potent PROTACs described at the time of publication (DC50 = 19 nM). It functions through a VHL-dependent and proteasome-dependent mechanism. [1] The (S,R,S)-AHPC-derived VHL ligand in HaloPROTACs was chosen because crystallographic evidence indicated solvent-exposed sites suitable for linker attachment. [1] HaloPROTAC3 is proposed as a useful chemical genetic tool for the controlled degradation of HaloTag7 fusion proteins in cells, offering an orthogonal approach to existing systems like Shield1. [1] |
| 分子式 |
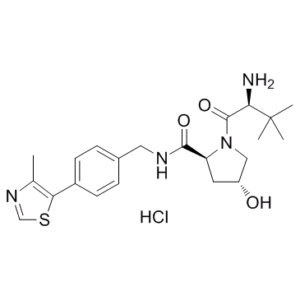
C₂₂H₃₁CLN₄O₃S
|
|
|---|---|---|
| 分子量 |
467.02
|
|
| 精确质量 |
466.18
|
|
| CAS号 |
1448189-80-7
|
|
| 相关CAS号 |
1448297-52-6;1448189-80-7 (HCl);
|
|
| PubChem CID |
118864076
|
|
| 外观&性状 |
White to yellow solid powder
|
|
| tPSA |
137
|
|
| 氢键供体(HBD)数目 |
4
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
31
|
|
| 分子复杂度/Complexity |
618
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
CC1=C(SC=N1)C2=CC=C(C=C2)CNC(=O)[C@@H]3C[C@H](CN3C(=O)[C@H](C(C)(C)C)N)O.Cl
|
|
| InChi Key |
JYRTWGCWUBURGU-MSSRUXLCSA-N
|
|
| InChi Code |
InChI=1S/C22H30N4O3S.ClH/c1-13-18(30-12-25-13)15-7-5-14(6-8-15)10-24-20(28)17-9-16(27)11-26(17)21(29)19(23)22(2,3)4;/h5-8,12,16-17,19,27H,9-11,23H2,1-4H3,(H,24,28);1H/t16-,17+,19-;/m1./s1
|
|
| 化学名 |
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.45 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (214.12 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1412 mL | 10.7062 mL | 21.4124 mL | |
| 5 mM | 0.4282 mL | 2.1412 mL | 4.2825 mL | |
| 10 mM | 0.2141 mL | 1.0706 mL | 2.1412 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Schematic depiction of a bifunctional HaloPROTAC containing chloroalkane (which binds HaloTag7 fusion proteins) and a hydroxyproline derivative which binds VHL.
Synthesis of HaloPROTACs containing Degradation Inducing Moiety A and Degradation Inducing Moiety B.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |
The average fluorescence per cell compared to vehicle control was measured by flow cytometry after 24 hour treatment with the indicated compounds and concentrations.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |
A) A study of linker length with Degradation Inducing Moiety B shows that three ethylene glycol units are optimal for the degradation of GFP-HaloTag7. B) Structures of HaloPROTACs that have weaker affinity for VHL. C) Reducing the affinity for VHL attenuates their ability to induce degradation of GFP-HaloTag7, although the effect is not necessarily linear.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |
A) The enantiomers of HaloPROTACs (containing D-amino acid residues) which do not bind VHL do not induce degradation of GFP-HaloTag7, supporting the necessity of VHL binding for activity. B) Pre-treatment with excessent-HaloPROTAC3 (1 hour) prevents degradation of GFP-HaloTag7 by HaloPROTAC3 after 24 hours. C) Pre-treatment with epoxomicin (4 hours) prevents degradation of GFP-HaloTag7 by HaloPROTAC3 after 20 hours. D)Treatment with VL285 attenuates the ability of HaloPROTAC3 to induce the degradation of GFP-HaloTag7. E) Structure of VL285. All error bars depict SEM.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |
A) Comparison of HaloPROTAC3 (quintuplicate) to Hyt36 (triplicate) shows that HaloPROTAC3 is significantly more potent and efficacious. B) HaloPROTAC3 leads to 50% degradation of GFP-HaloTag7 within 4 to 8 hours. C) Significant recovery from 24 hour treatment with HaloPROTAC3 is observed after a 24 hour washout.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |
Fluorescent microscopy shows drastic loss of fluorescence upon 24 hour treatment with HaloPROTAC3 but not the inactiveent-HaloPROTAC3.
Immunoblotting confirms that nearly complete degradation of A) GFP-HaloTag7 is observed after 24 hour treatment with 500 nM HaloPROTAC3, with significant degradation at 50 nM HaloPROTAC3. HaloPROTAC3 can lead to degradation of other HaloTag7 fusion proteins such as B) HaloTag7-ERK1 and HaloTag7-MEK1. As expected, endogenous ERK and MEK are not degraded.ACS Chem Biol.2015 Aug 21;10(8):1831-7. |