| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
Lck (IC50 = 249 nM); PAK5 (IC50 = 166 nM)
Spleen Tyrosine Kinase (Syk) (recombinant human Syk, IC50 = 1.6 nM); >300-fold selectivity over Lyn (IC50 = 520 nM), Src (IC50 = 610 nM), JAK2 (IC50 = 750 nM); no activity against EGFR, Abl (IC50 > 1000 nM) [1] - Confirmed Syk as primary target (rheumatoid arthritis model; no additional IC50 values; consistent with [1]’s selectivity) [2] |
|---|---|
| 体外研究 (In Vitro) |
PRT062607 (P505-15) 是一种全新、极其有效、强效的口服 Syk 小分子抑制剂。使用两种不同的纯激酶测定来研究 PRT062607 对其靶激酶 Syk 的功效。根据 FRET 测试,半最大 Syk 抑制需要 6±0.2 nM(平均值±SEM)。放射性酶测定得出的 Syk IC50 为 2.1±0.4 nM(平均值±SEM),表明效力相似。 PRT062607 有效抑制人全血中 Fcε 受体 1 介导的嗜碱性粒细胞脱颗粒 (IC50 0.15 μM) 和 B 细胞抗原受体介导的 B 细胞信号传导和激活(IC50 分别为 0.27 和 0.28 μM)[1]。
抑制CLL细胞增殖:原代人CLL细胞(IC50 = 8.3 nM);50 nM PRT062607 (P505-15, BIIB057) HCl处理72小时,CLL细胞活力降低68%;Western blot显示p-Syk(Tyr525/526)和p-PLCγ2(Tyr759)分别下调92%/88%[1] - 与氟达拉滨协同作用:20 nM PRT062607 HCl + 1 μM氟达拉滨(CLL标准药物)处理48小时,CLL细胞凋亡率从氟达拉滨单药的35%升至68%;协同指数(CI)=0.45(呈协同效应)[1] - 抑制B细胞活化:15 nM PRT062607 HCl处理小鼠脾B细胞72小时,抗IgM诱导的增殖减少85%;流式细胞术检测显示活化标志物CD69表达降低82%[1] - 抑制白细胞介导的炎症:100 nM PRT062607 HCl处理人单核细胞24小时,LPS诱导的TNF-α/IL-6分泌减少78%/75%;Transwell实验显示中性粒细胞趋化性抑制70%[2] |
| 体内研究 (In Vivo) |
当对 CAIA 模型中的小鼠口服PRT062607 (P505-15) 时,与载体对照相比,通过每日炎症评分确定,爪子炎症平均减少了 12%、44% 和 87%。研究结束时,平均血浆浓度(24 小时内的 C 平均值)确定为 0.38、0.95 和 1.47 μM。给予 30 mg/kg PRT062607 的小鼠表现出关节退化显着减少,以至于无法将小鼠与正常小鼠区分开。到研究结束时,大鼠 CIA 模型中的大多数动物(八分之七)(平均炎症评分±SEM=0.63±1.1;与媒介物相比,p<0.0001)由于高剂量的PRT062607(15 毫克/千克,每日两次)[1]。
在人全血中,PRT062607 (P505-15) 能有效抑制B细胞抗原受体介导的B细胞信号传导和激活(IC50分别为0.27和0.28 μM)和fce1受体介导的嗜碱性粒细胞脱粒(IC50分别为0.15 μM)。给药后,在小鼠体内测量了相似的体外抑制水平(Syk信号IC50 0.32 μM)。在更高的浓度下,syk独立的信号传导和激活不受影响,这表明激酶抑制在细胞系统中的特异性。口服P505-15在两种类风湿关节炎啮齿动物模型中产生剂量依赖性抗炎活性。在特异性抑制Syk活性约67%的浓度下,观察到统计学上显著的疗效。因此,特异性Syk抑制可以模拟Syk基因缺陷来调节免疫功能,为PRT062607 (P505-15) 治疗人类疾病提供了一种治疗策略。[2] 携带原代人CLL异种移植瘤的NSG小鼠([1]):口服PRT062607 HCl(25 mg/kg/天)持续28天,外周血CLL细胞计数较溶剂组减少82%;与氟达拉滨(10 mg/kg/3天,腹腔注射)联用时,CLL细胞计数减少95%[1] - 大鼠胶原诱导关节炎(CIA)模型([2]):口服PRT062607 HCl(30 mg/kg/天)持续21天,关节炎评分从溶剂组8.5降至2.1;踝关节炎症浸润减少75%(组织病理学);血清IL-6/TNF-α分别降低70%/68%[2] - 小鼠B细胞依赖抗体反应模型([1]):单次口服PRT062607 HCl(40 mg/kg),免疫后7天绵羊红细胞(SRBC)特异性IgG生成减少65%[1] |
| 酶活实验 |
SYK Autophosphorylation/SYK自身磷酸化。[1]
采用PRT062607 (P505-15) 或不用对Ramos细胞进行预孵育30分钟(每实验107个细胞),然后用1 μg/ml抗igm在37℃下刺激30分钟。通过将细胞重悬在含有新鲜蛋白酶和磷酸酶抑制剂的RIPA裂解缓冲液(50 mM Tris 7.4, 1% NP40, 0.5%脱氧胆酸钠,150 mM NaCl, 0.5 mM EDTA)中终止信号传导,并在冰上孵育1小时。离心后,蛋白裂解物用蛋白A/G糖珠预清。裂解液与兔抗syk抗体孵育过夜(4℃),用蛋白A/G sepharose beads沉淀。洗涤珠变性,10% SDS-PAGE溶解蛋白,兔抗syk抗体免疫印迹,剥离,兔抗psyk Y526/526抗体重新印迹。 Syk激酶活性实验(文献1/2):重组人Syk激酶结构域(50 ng/孔)与PRT062607 HCl(0.01-100 nM)在反应缓冲液(25 mM HEPES pH 7.5,10 mM MgCl₂,1 mM DTT,0.1 mM 钒酸钠)中于37°C孵育20分钟。加入10 μM ATP和荧光标记肽底物(序列:biotin-GGEEEEYFELVAKKKK),30°C继续孵育60分钟。链霉亲和素包被的96孔板捕获磷酸化底物,抗磷酸酪氨酸抗体检测,均相时间分辨荧光(HTRF,激发光340 nm,发射光665 nm)定量激酶活性;非线性回归分析计算IC50[1][2] |
| 细胞实验 |
细胞内磷酸流式细胞术。[1]
Ramos细胞(0.5 × 106)悬浮于200 μl新鲜培养基(RPMI + 10%胎牛血清)中,用载药或PRT062607 (P505-15) 预处理(37℃下30分钟)。细胞不受刺激或以1 μg/ml goat F(ab) ' 2 anti-IgM (Life Technologies)刺激10分钟。从CLL患者(n = 7)外周血中获得的Ficoll纯化(2 × 106)冷冻活CLL细胞,37℃解冻,用10 ml RPMI培养基加10%胎牛血清离心洗涤,以106细胞/ml的浓度在相同培养基中重悬。在0.3 mM H2O2刺激30分钟前,等量(200 μl)细胞用载药或PRT062607 (P505-15) 处理(Reth 2002;Irish et al., 2006) (8.8 M高汤),然后加入6 μg山羊F(ab’)2抗人IgG和抗人IgM 1:1的混合物10分钟。加入60 μl的16%多聚甲醛溶液,室温孵育10分钟,阻断信号传导。将固定的细胞在冷冻的磷酸盐缓冲盐水(PBS)中离心洗涤2次,悬浮在预冷至- 20°C的50%甲醇溶液中,4°C保存过夜。按照制造商的说明,通过在含有1% BSA的PBS中洗涤渗透化细胞两次,然后在含有各种抗体的同一缓冲液中孵育,对细胞进行细胞内磷酸流式细胞术染色。在FACS分析之前,细胞在含有1% BSA的PBS中再次洗涤,其中使用BD Biosciences FACS Calibur收集了至少2000个事件。使用FlowJo软件对数据进行分析。 细胞凋亡。[1] 采用pe偶联单克隆活性caspase-3抗体凋亡试剂盒检测细胞凋亡。将细胞悬浮在生长培养基(0.5 × 106个细胞/ml)中,并在FACS分析前用指示浓度的PRT062607 (P505-15) 或对照处理72小时。在一些实验中,将SU-DHL6 (0.5 × 106个细胞)与100µl肝素化人全血混合。样品用1µM PRT062607 (P505-15) 处理24小时,然后用抗人CD19抗体表面染色,准备用于FACS分析活性caspase-3。 原代人CLL细胞实验[1]:从患者外周血分离CLL细胞,接种于96孔板(2×10⁵个/孔)。用PRT062607 HCl(1-50 nM)单药或联合氟达拉滨(0.1-5 μM)处理72小时。MTT法检测活力;30 μg蛋白经10% SDS-PAGE分离,Western blot检测p-Syk/p-PLCγ2水平[1] - 小鼠B细胞活化实验[1]:从C57BL/6小鼠分离脾B细胞,接种于96孔板(4×10³个/孔)。PRT062607 HCl(5-200 nM)预处理1小时后,抗小鼠IgM(10 μg/mL)刺激72小时。[³H]-胸腺嘧啶掺入法检测增殖;FITC标记抗CD69抗体流式分析CD69表达[1] - 人单核细胞炎症实验[2]:外周血单核细胞接种于24孔板(1×10⁵个/孔),PRT062607 HCl(20-500 nM)处理2小时后,LPS(1 μg/mL)刺激24小时。收集上清液,ELISA检测TNF-α/IL-6;Transwell实验中,下室加入处理后的单核细胞,上室加入中性粒细胞,4小时后计数迁移的中性粒细胞[2] |
| 动物实验 |
10, 15, or 20 mg/kg; Oral administration
NOD/SCID mice injected with Ramos cells In Vitro and In Vivo Stimulation with Anti-IgD. [1] Spleens were harvested from Balb/c mice and separated into a single cell suspension using a single cell strainer. Cells were collected in PBS containing 1% BSA by centrifugation, washed once in the same buffer, and resuspended in RPMI (containing 10% FCS) at 106 cells/ml. Aliquots (190 µl) were then treated with various concentrations of PRT062607 (P505-15) for 1 hour prior to stimulating overnight in a 37°C tissue culture incubator with 1 µl anti-mouse immunoglobulin D (IgD). Following stimulation, cells were stained with anti-CD80/86 FITC and anti-CD45R/B220 APC for 30 minutes at room temperature, washed once in PBS containing 1% BSA, and resuspend in 300 μl of the same buffer for collection of 2000 CD45-positive events by flow cytometry. Balb/c mice (n = 5 per group) received daily oral BID doses of vehicle (0.5% methylcellulose in water) or PRT062607 (P505-15) (15 mg/kg) for a total of 5 days. On study day 1, 1 hour after the first oral dose, mice received a single 200-μl subcutaneous injection of control goat serum or anti-IgD serum. On study day 5, mice were anesthetized with SC ketamine cocktail and exsanguinated via cardiac puncture. Spleens were weighed and then sectioned and stained with hematoxylin and eosin for histology. Xenograft Studies. [1] NOD/SCID mice were acclimated in-house at least 3 days prior to use. Ramos cells (3 × 106) were injected subcutaneously into the hind flank area of conscious mice using a 27-gauge needle in an injection volume of less than 0.5 ml. Following injection, mice were randomized into treatment groups (n = 15) and dosed twice daily by oral gavage with vehicle (0.5% methylcellulose in water) or 10, 15, or 20 mg/kg PRT062607 (P505-15) . Body weights were obtained at least once per week, and caliper measurements of tumors were determined twice per week beginning when palpable tumors were formed until the end of the study. Tumor volume was assessed by caliper measurement using a formula [maximum length × width × height × pi/6]. Twice daily dosing of vehicle or PRT062607 (P505-15) continued until the vehicle or any treatment group exhibited tumors that exceeded 1.5 g in size. At the time of termination (5 weeks post-Ramos inoculation) the mice were anesthetized with a ketamine cocktail. A blood sample via cardiac puncture was obtained for CBC and plasma concentration determination, and then the mice were euthanized via cervical dislocation. Tumors were excised, weighed, and then snap frozen in liquid nitrogen for determination of concentration of PRT062607 (P505-15) in the tumor tissue. Statistical comparison of tumor weights across groups was performed using a t-test. CLL xenograft model (NSG mice, [1]): 6-week-old female NSG mice were intravenously injected with 5×10⁶ primary human CLL cells. 7 days later, mice were randomized to: vehicle, PRT062607 HCl (25 mg/kg/day, oral gavage), or combination (25 mg/kg/day PRT062607 HCl + 10 mg/kg fludarabine, i.p. every 3 days). Treatments lasted 28 days; PRT062607 HCl was dissolved in 0.5% methylcellulose + 0.2% Tween 80. Peripheral blood CLL cells were counted via flow cytometry (anti-CD5/CD19 antibodies) [1] - Rat CIA model (Lewis rats, [2]): Arthritis was induced by intradermal injection of bovine type II collagen (100 μg/rat) emulsified in complete Freund’s adjuvant. 14 days post-induction, rats received PRT062607 HCl (30 mg/kg/day, oral gavage) for 21 days. Drug was dissolved in 0.5% methylcellulose; arthritis score (0-10, based on joint redness/swelling) was recorded every 3 days. At study end, ankle joints were collected for histopathology, and serum cytokines were measured via ELISA [2] |
| 药代性质 (ADME/PK) |
In mice (literature 1): Oral bioavailability of PRT062607 HCl = 58% (25 mg/kg dose); plasma half-life (t₁/₂) = 4.1 hours; maximum plasma concentration (Cmax) = 4.6 μM at 1.2 hours post-oral administration [1]
- Plasma protein binding: 99.2% binding to human plasma proteins and 98.8% binding to mouse plasma proteins (measured via ultrafiltration method) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In 28-day CLL study ([1]): No significant weight loss (>8%); serum ALT (26 ± 3 U/L), AST (49 ± 5 U/L), BUN (17 ± 2 mg/dL) were within normal ranges [1]
- In 21-day CIA study ([2]): 1/10 rats showed mild gastrointestinal discomfort (resolved by day 7); no histopathological changes in liver, kidney, or joint tissues [2] |
| 参考文献 |
|
| 其他信息 |
Given its preclinical activity and specificity, especially when compared with previously studied SYK inhibitors, P505-15 is an attractive compound for clinical development. Additional studies aimed at defining the biologic characteristics associated with SYK sensitivity are needed. Exploring additional P505-15 combinations including chemotherapy, monoclonal antibodies, or novel combinations of kinase inhibitors targeting multiple branches of signaling pathways, may identify further therapeutic opportunities. A dose finding study using P505-15 in healthy volunteers has been completed and includes single and multiple dosing regimens.[1]
B-cell receptor (BCR) associated kinases including spleen tyrosine kinase (SYK) contribute to the pathogenesis of B-cell malignancies. SYK is persistently phosphorylated in a subset of non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL), and SYK inhibition results in abrogation of downstream kinase activity and apoptosis. P505-15 (also known as PRT062607) is a novel, highly selective, and orally bioavailable small molecule SYK inhibitor (SYK IC(50) = 1 nM) with anti-SYK activity that is at least 80-fold greater than its affinity for other kinases. We evaluated the preclinical characteristics of P505-15 in models of NHL and CLL. P505-15 successfully inhibited SYK-mediated B-cell receptor signaling and decreased cell viability in NHL and CLL. Oral dosing in mice prevented BCR-mediated splenomegaly and significantly inhibited NHL tumor growth in a xenograft model. In addition, combination treatment of primary CLL cells with P505-15 plus fludarabine produced synergistic enhancement of activity at nanomolar concentrations. Our findings support the ongoing development of P505-15 as a therapeutic agent for B-cell malignancies. A dose finding study in healthy volunteers has been completed.[2] PRT062607 (P505-15, BIIB057) HCl is a potent, selective, orally available spleen tyrosine kinase (Syk) inhibitor, developed for B-cell malignancies (e.g., chronic lymphocytic leukemia) and autoimmune diseases (e.g., rheumatoid arthritis) [1][2] - Its mechanism of action involves specific inhibition of Syk autophosphorylation, blocking downstream B-cell receptor (BCR) signaling (for CLL) and Fc receptor-mediated leukocyte activation (for inflammation) [1][2] - It exhibits synergistic antitumor activity with fludarabine in CLL, potentially improving treatment efficacy for chemo-responsive malignancies [1] |
| 分子式 |
C19H23N9O.HCL
|
|---|---|
| 分子量 |
429.91
|
| 精确质量 |
429.179
|
| 元素分析 |
C, 53.08; H, 5.63; Cl, 8.25; N, 29.32; O, 3.72
|
| CAS号 |
1370261-97-4
|
| 相关CAS号 |
PRT062607;1370261-96-3
|
| PubChem CID |
56948527
|
| 外观&性状 |
Light yellow to khaki solid powder
|
| LogP |
3.463
|
| tPSA |
153.88
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
544
|
| 定义原子立体中心数目 |
2
|
| SMILES |
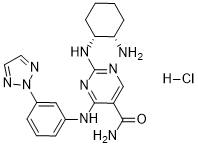
C1CC[C@H]([C@H](C1)N)NC2=NC=C(C(=N2)NC3=CC(=CC=C3)N4N=CC=N4)C(=O)N.Cl
|
| InChi Key |
RMNLLPXCNDZJMJ-IDVLALEDSA-N
|
| InChi Code |
InChI=1S/C19H23N9O.ClH/c20-15-6-1-2-7-16(15)26-19-22-11-14(17(21)29)18(27-19)25-12-4-3-5-13(10-12)28-23-8-9-24-28;/h3-5,8-11,15-16H,1-2,6-7,20H2,(H2,21,29)(H2,22,25,26,27);1H/t15-,16+;/m0./s1
|
| 化学名 |
4-((3-(2H-1,2,3-triazol-2-yl)phenyl)amino)-2-(((1R,2S)-2-aminocyclohexyl)amino)pyrimidine-5-carboxamide hydrochloride
|
| 别名 |
P505-15; P-50515; PRT2607; PRT062607; P50515; BIIB057; PRT-062607; PRT 062607; PRT-2607; PRT 2607; 1370261-96-3; PRT-062607; 2-[[(1R,2S)-2-aminocyclohexyl]amino]-4-[3-(triazol-2-yl)anilino]pyrimidine-5-carboxamide; K9C42672RH; P505-15; BIIB057; UNII-K9C42672RH; P-505-15; P 505-15; P 50515; BIIB-057; BIIB 057.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.82 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.82 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.82 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: Saline:30mg/mL 配方 5 中的溶解度: 50 mg/mL (116.30 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3261 mL | 11.6303 mL | 23.2607 mL | |
| 5 mM | 0.4652 mL | 2.3261 mL | 4.6521 mL | |
| 10 mM | 0.2326 mL | 1.1630 mL | 2.3261 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT01652937 | Withdrawn | Drug: BIIB057 Drug: Placebo |
Rheumatoid Arthritis | Biogen | August 2012 | Phase 2 |
P505-15 selectively inhibits proliferation of Syk-dependent B cell lines.J Pharmacol Exp Ther.2012 Feb;340(2):350-9. |
P505-15 attenuates antibody-induced inflammation in a mouse model of rheumatoid arthritis.J Pharmacol Exp Ther.2012 Feb;340(2):350-9. |
Oral administration of P505-15 significantly ameliorates the severity and development of arthritis in the rat CIA model.J Pharmacol Exp Ther.2012 Feb;340(2):350-9. |