| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
Histamine H2 receptor
|
|---|---|
| 体外研究 (In Vitro) |
体外活性:雷尼替丁使肝细胞对活化中性粒细胞的细胞毒性产物的杀伤敏感,而法莫替丁缺乏这种能力。雷尼替丁可抑制体外脂多糖刺激的单核细胞中肿瘤坏死因子-α (TNF-α) 的产生。雷尼替丁剂量依赖性地降低吗啡的 Kel,最大效果为 50%,并增加离体豚鼠肝细胞中 6-葡萄糖醛酸吗啡与 3-葡萄糖醛酸吗啡的相对浓度。雷尼替丁逐渐降低吗啡-3-葡萄糖醛酸/吗啡-6-葡萄糖醛酸的比例,最高可达 21%。
|
| 体内研究 (In Vivo) |
雷尼替丁导致大鼠肝损伤,证据是大鼠服用雷尼替丁后 6 小时内血清丙氨酸转氨酶、天冬氨酸转氨酶和 γ-谷氨酰转移酶活性增加。雷尼替丁可抑制大鼠肝缺血/再灌注引起的肝组织 TNF-α、细胞因子诱导的中性粒细胞趋化剂水平的增加以及中性粒细胞的肝脏积聚。雷尼替丁联合治疗可增强 LPS 诱导的肝损伤前的凝血,而抗凝剂可减轻 LPS/RAN 治疗大鼠的肝损伤。雷尼替丁/LPS治疗的大鼠导致肝窦中纤维蛋白凝块的形成,并防止与减少肝细胞损伤相关的纤维蛋白沉积。雷尼替丁联合治疗可增强大鼠肝细胞损伤发生前 LPS 诱导的 TNF 增加。雷尼替丁在高架十字迷宫中显示出抗焦虑作用,这通过大鼠张开臂的时间增加、更多的张开臂扫描和更多的末端偏移来表明。
|
| 细胞实验 |
在离体豚鼠肝细胞中研究雷尼替丁对吗啡代谢的影响,特别强调吗啡-3-葡糖苷和吗啡-6-葡糖苷的比值。雷尼替丁对吗啡的剂量依赖性最大可降低50%,使吗啡-6-葡糖苷的相对浓度增加到吗啡-3-葡糖苷的相对浓度。这些影响可能是由于对所涉及的偶联酶的直接或间接影响,或对吗啡或葡萄糖醛酸盐跨细胞膜运输的影响。后一种解释被拒绝,因为观察到吗啡、吗啡-3-葡糖苷和吗啡-6-葡糖苷细胞内和细胞外浓度的比值不受雷尼替丁的影响。雷尼替丁浓度的增加使吗啡-3-葡糖苷/吗啡-6-葡糖苷的比值逐渐降低了21%。这可能源于能量或共底物供应的干扰,或通过对不同UDPGTases的直接影响。观察到,目前对吗啡葡糖醛酸化的影响与给予已知的共底物(UDPGA)消耗物时所观察到的相反,表明雷尼替丁的作用很可能是直接抑制尿苷5'-二磷酸葡糖醛酸转移酶,对负责3'-葡糖醛酸化的同功酶的作用更为明显。[3]
|
| 动物实验 |
Drug idiosyncrasy is an adverse event of unknown etiology that occurs in a small fraction of people taking a drug. Some idiosyncratic drug reactions may occur from episodic decreases in the threshold for drug hepatotoxicity. Previous studies in rats have shown that modest underlying inflammation triggered by bacterial lipopolysaccharide (LPS) can decrease the threshold for xenobiotic hepatotoxicity. The histamine-2 (H2)-receptor antagonist ranitidine (RAN) causes idiosyncratic reactions in people, with liver as a usual target. Researchers tested the hypothesis that RAN could be rendered hepatotoxic in animals undergoing a modest inflammatory response.
[1]
Male rats were treated with a nonhepatotoxic dose of LPS (44 x 10(6) endotoxin units/kg i.v.) or its vehicle and then 2 h later with a nonhepatotoxic dose of RAN (30 mg/kg i.v.) or its vehicle. Liver injury was evident only in animals treated with both RAN and LPS as estimated by increases in serum alanine aminotransferase, aspartate aminotransferase, and gamma-glutamyl transferase activities within 6 h after RAN administration. LPS/RAN cotreatment resulted in midzonal liver lesions characterized by acute necrosuppurative hepatitis. Famotidine (FAM) is an H2-antagonist for which the propensity for idiosyncratic reactions is far less than RAN. Rats given LPS and FAM at a dose pharmacologically equipotent to that of RAN did not develop liver injury. In vitro, RAN sensitized hepatocytes to killing by cytotoxic products from activated neutrophils, whereas FAM lacked this ability. The results indicate that a response resembling human RAN idiosyncrasy can be reproduced in animals by RAN exposure during modest inflammation.[1] Researchers previously reported that ranitidine, an H(2) receptor antagonist, inhibited neutrophil activation in vitro and in vivo, contributing to reduce stress-induced gastric mucosal injury in rats. In this study, Researchers examined whether ranitidine would reduce ischemia/reperfusion-induced liver injury, in which activated neutrophils are critically involved, in rats. Researchers also examined the effect of famotidine, another H(2) receptor antagonist, on leukocyte activation in vitro and after ischemia/reperfusion-induced liver injury in rats to know whether inhibition of neutrophil activation by ranitidine might be dependent on its blockade of H(2) receptors. Ranitidine inhibited the activation of neutrophils in vitro as reported previously, whereas famotidine significantly enhanced it. Ranitidine inhibited the production of tumor necrosis factor-alpha (TNF-alpha) in monocytes stimulated with lipopolysaccharide in vitro, whereas famotidine did not. Although hepatic ischemia/reperfusion-induced increases in hepatic tissue levels of TNF-alpha, cytokine-induced neutrophil chemoattractant, and hepatic accumulation of neutrophils were inhibited by intravenously administered 30 mg/kg ranitidine, these increases were significantly enhanced by 5 mg/kg i.v. famotidine. The decreases in both hepatic tissue blood flow and bile secretion and the increases in serum levels of transaminases seen after reperfusion were significantly inhibited by ranitidine, whereas these changes were more marked in animals given famotidine than in controls. These observations strongly suggested that ranitidine could reduce ischemia/reperfusion-induced liver injury by inhibiting neutrophil activation directly, or indirectly by inhibiting the production of TNF-alpha, which is a potent activator of neutrophils. Furthermore, the therapeutic efficacy of ranitidine might not be explained solely by its blockade of H(2) receptor.[2] |
| 药代性质 (ADME/PK) |
Metabolism / Metabolites
Ranitidine has known human metabolites that include Desmethylranitidine. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Although interpatient variability exists, the dose of ranitidine in breastmilk is less than the dose used in newborn infants. However, the finding that ranitidine spontaneously breaks down to a cancer-causing chemical caused its removal from the market in the US and other countries. Other drugs are recommended. ◉ Effects in Breastfed Infants One 54-day-old breastfed infant had no observable adverse effects after maternal ingestion of ranitidine 150 mg every 12 hours for 2 days. ◉ Effects on Lactation and Breastmilk Histamine H2-receptor blockade is known to stimulate prolactin secretion. Ranitidine in intravenous doses over 100 mg or during long-term oral use have increased serum prolactin in some studies, and rare cases of gynecomastia have been reported. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. |
| 参考文献 | |
| 其他信息 |
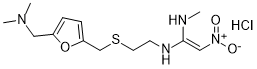
Ranitidine is a member of the class of furans used to treat peptic ulcer disease (PUD) and gastroesophageal reflux disease. It has a role as an anti-ulcer drug, a H2-receptor antagonist, an environmental contaminant, a xenobiotic and a drug allergen. It is a member of furans, a tertiary amino compound, a C-nitro compound and an organic sulfide.
Ranitidine is a member of the class of histamine H2-receptor antagonists with antacid activity. Ranitidine is a competitive and reversible inhibitor of the action of histamine, released by enterochromaffin-like (ECL) cells, at the histamine H2-receptors on parietal cells in the stomach, thereby inhibiting the normal and meal-stimulated secretion of stomach acid. In addition, other substances that promote acid secretion have a reduced effect on parietal cells when the H2 receptors are blocked. Ranitidine Hydrochloride is a member of the class of histamine H2-receptor antagonists with antacid activity. Ranitidine is a competitive and reversible inhibitor of the action of histamine, released by enterochromaffin-like (ECL) cells, at the histamine H2-receptors on parietal cells in the stomach, thereby inhibiting the normal and meal-stimulated secretion of stomach acid. In addition, other substances that promote acid secretion have a reduced effect on parietal cells when the H2 receptors are blocked. A non-imidazole blocker of those histamine receptors that mediate gastric secretion (H2 receptors). It is used to treat gastrointestinal ulcers. See also: Ranitidine (annotation moved to). Exposure to a nontoxic dose of bacterial lipopolysaccharide (LPS) increases the hepatotoxicity of the histamine-2 (H2) receptor antagonist, ranitidine (RAN). Because some of the pathophysiologic effects associated with LPS are mediated through the expression and release of inflammatory mediators such as tumor necrosis factor alpha (TNF), this study was designed to gain insights into the role of TNF in LPS/RAN hepatotoxicity. To determine whether RAN affects LPS-induced TNF release at a time near the onset of liver injury, male Sprague-Dawley rats were treated with 2.5 x 10(6) endotoxin units (EU)/kg LPS or its saline vehicle (iv) and 2 h later with either 30 mg/kg RAN or sterile phosphate-buffered saline vehicle (iv). LPS administration caused an increase in circulating TNF concentration. RAN cotreatment enhanced the LPS-induced TNF increase before the onset of hepatocellular injury, an effect that was not produced by famotidine, a H2-receptor antagonist without idiosyncrasy liability. Similar effects were observed for serum interleukin (IL)-1beta, IL-6, and IL-10. To determine if TNF plays a causal role in LPS/RAN-induced hepatotoxicity, rats were given either pentoxifylline (PTX; 100 mg/kg, iv) to inhibit the synthesis of TNF or etanercept (Etan; 8 mg/kg, sc) to impede the ability of TNF to reach cellular receptors, and then they were treated with LPS and RAN. Hepatocellular injury, the release of inflammatory mediators, hepatic neutrophil (PMN) accumulation, and biomarkers of coagulation and fibrinolysis were assessed. Pretreatment with either PTX or Etan resulted in the attenuation of liver injury and diminished circulating concentrations of TNF, IL-1beta, IL-6, macrophage inflammatory protein-2, and coagulation/fibrinolysis biomarkers in LPS/RAN-cotreated animals. Neither PTX nor Etan pretreatments altered hepatic PMN accumulation. These results suggest that TNF contributes to LPS/RAN-induced liver injury by enhancing inflammatory cytokine production and hemostasis.[4] This study examined the effects of the H1-antagonist chlorpheniramine and the H2-antagonist ranitidine on reinforcement and anxiety-parameters following unilateral injection into the vicinity of the nucleus basalis magnocellularis (NBM). In Experiment 1, rats with chronically implanted cannulae were injected with chlorpheniramine or ranitidine (each at doses of 0.1, 1, 10 and 20 microg) and were placed into one of four restricted quadrants of a circular open field (closed corral) for a single conditioning trial. During the test for conditioned corral preference, when provided a choice between the four quadrants, only those rats injected with 10 or 20 microg chlorpheniramine spent more time in the treatment corral, indicative of a positively reinforcing action. None of the other doses of chlorpheniramine or of the H2-antagonist influenced rats' preference behavior. In Experiment 2, the elevated plus-maze (EPM) was used to gauge possible anxiolytic or anxiogenic effects of intra-basalis injection of chlorpheniramine or ranitidine (each at doses of 0.1, 1, 10 and 20 microg). A single injection of chlorpheniramine at 0.1 or 20 microg as well as ranitidine at 20 microg was found to exert anxiolytic-like effects in the EPM. Both compounds elevated the time spent on the open arms and increased scanning over the edge of an open arm. None of the other doses of the H1- and H2-antagonist influenced rats' behavior in the EPM. In sum, these findings show that H1- and H2-receptor antagonists differentially modulate reinforcement and fear-related processes in the NBM and thus, provide the first evidence for a behavioral relevance for the histaminergic innervation of this brain site.[5] |
| 分子式 |
C13H23CLN4O3S
|
|
|---|---|---|
| 分子量 |
350.86
|
|
| 精确质量 |
350.117
|
|
| 元素分析 |
C, 44.50; H, 6.61; Cl, 10.10; N, 15.97; O, 13.68; S, 9.14
|
|
| CAS号 |
66357-59-3
|
|
| 相关CAS号 |
Ranitidine-d6 hydrochloride; 1185238-09-8; Ranitidine; 66357-35-5; Ranitidine bismuth citrate; 128345-62-0; 66357-59-3 (HCl); 71130-06-8 (HCl)
|
|
| PubChem CID |
3001055
|
|
| 外观&性状 |
Off-white to yellow solid powder
|
|
| 熔点 |
134°C (dec.)
|
|
| LogP |
3.566
|
|
| tPSA |
111.56
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
9
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
347
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
S(C([H])([H])C([H])([H])N([H])/C(=C(\[H])/[N+](=O)[O-])/N([H])C([H])([H])[H])C([H])([H])C1=C([H])C([H])=C(C([H])([H])N(C([H])([H])[H])C([H])([H])[H])O1
|
|
| InChi Key |
GGWBHVILAJZWKJ-KJEVSKRMSA-N
|
|
| InChi Code |
InChI=1S/C13H22N4O3S.ClH/c1-14-13(9-17(18)19)15-6-7-21-10-12-5-4-11(20-12)8-16(2)3;/h4-5,9,14-15H,6-8,10H2,1-3H3;1H/b13-9+;
|
|
| 化学名 |
(E)-1-N'-[2-[[5-[(dimethylamino)methyl]furan-2-yl]methylsulfanyl]ethyl]-1-N-methyl-2-nitroethene-1,1-diamine;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (5.93 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.93 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.93 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 110 mg/mL (313.52 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8501 mL | 14.2507 mL | 28.5014 mL | |
| 5 mM | 0.5700 mL | 2.8501 mL | 5.7003 mL | |
| 10 mM | 0.2850 mL | 1.4251 mL | 2.8501 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Histamine Release and Implications of H1- and H2- Blockade in Adult Cardiac Surgery - A Randomised Controlled Study
CTID: null
Phase: Phase 4 Status: Prematurely Ended
Date: 2005-04-27