| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg | |||
| Other Sizes |
| 靶点 |
CGRP receptor ( Ki = 0.027 nM )
|
|---|---|
| 体外研究 (In Vitro) |
Rimegepant (BMS-927711, BHV-3000) 是一种有效的、竞争性的、选择性的人降钙素基因相关肽 (CGRP) 受体拮抗剂,ki 为 0.027 nM。
|
| 体内研究 (In Vivo) |
体内疗效[1]
开发了一种新的无创绒猴恢复模型,用于评估CGRP受体拮抗剂的体内疗效,该模型利用激光多普勒面部血流作为颅内动脉直径的替代指标。简而言之,对狨猴进行麻醉,并通过四次静脉注射hαCGRP(10μg/kg)来增加面部血流量,每45分钟(-30、15、60和105分钟)一次。通过激光多普勒血流仪测量0分钟皮下注射(SC)拮抗剂对hαCGRP诱导的面部血流变化的影响。在该模型中,化合物8(Rimegapant,也称为BMS-927711)抑制了皮下(SC)给药时hαCGRP诱导的狨猴面部血流量的增加。与给药前hCGRP对照组(-30分钟)相比,在给药后15、60和105分钟,以7mg/kg的剂量皮下注射化合物8,观察到对CGRP诱导的面部血流作用的强烈(>50%)抑制(图2)。比较给药后15分钟的活性与暴露量,约400 nM的血浆水平与强烈的体内疗效(>65%的抑制率)相关。相比之下,在该模型中,需要5 nM以上的血浆水平才能达到类似的疗效。在给药后60和105分钟,8的峰值抑制在75-80%时非常强烈,相应的血浆水平略低于800 nM(图3) 体内PK[1] 化合物8在大鼠(FPO=45%)和食蟹猴(溶液FPO=67%)中表现出良好的口服生物利用度。当以晶体材料的游离碱悬浮液给药时,8在猴子体内的口服生物利用度保持在48%。 |
| 酶活实验 |
[125I]CGRP结合试验的竞争:[1]
使用放射性配体竞争分析法测量8抑制放射性标记的人α-CGRP([125I]CGRP)与人CGRP受体结合的能力。内源性表达CGRP受体的人神经母细胞瘤SK-N-MC细胞被用作CGRP受体来源(Aiyar等人,2001)。首先将8溶解并连续稀释在100%DMSO中。将化合物进一步稀释25倍到测定缓冲液(50 mM Tris-Cl pH 7.5,5 mM MgCl2,0.005%Triton X-100)中,并转移(50µl)到96孔测定板中。[125I]在测定缓冲液中将CGRP稀释至72 pM,并向每个孔中加入50µl(测定中的最终浓度为18 pM,Kd=27.7 pM)。将SK-N-MC膜颗粒解冻,用新鲜的0.1%哺乳动物蛋白酶抑制剂混合物在测定缓冲液中稀释,并均质化。然后向每个孔中加入100µl的匀浆(5至10µg蛋白质)。将测定板在室温(25°C)下孵育两小时。通过立即加入过量的冷洗涤缓冲液(50mM Tris-Cl pH 7.5,0.1%BSA)终止测定,然后用预浸在0.5%PEI中的玻璃纤维过滤器过滤。非特异性结合被定义为在1µMβ-CGRP存在下的结合。使用伽马闪烁计数器测量蛋白质结合放射性。IC50定义为抑制50%放射性配体结合所需的化合物浓度。 其他物种中的CGRP受体结合[1] 对S6 8在绒猴(普通绒猴(Callithrix jacchus))CGRP受体上的效力进行了测试。放射性配体结合分析用于评估活性:使用每种物种的50µg脑匀浆作为受体来源,15pM[125I]CGRP作为放射性配体。 使用人重组酶抑制CYP[1] 使用3-氰基-7-乙氧基香豆素(CYP1A2和CYP2C19)、7-甲氧基-4-三氟甲基香豆素(CYP2C9)或3-[2-(N,N-二乙基-N-甲基氨基)乙基]-7-甲氧基-4-甲基香豆素(CY2D6)作为模型底物,测量测试化合物抑制由杆状病毒感染的昆虫细胞制备的微粒体中cDNA衍生的CYP酶的能力。CYP3A4用两种底物7-苄氧基-4-三氟甲基香豆素(BFC)和苯甲酰间苯二酚进行了测试。对每种酶测定试验化合物的50%抑制浓度(IC50)。将每种模型底物的单一浓度(约为表观Km,BFC除外)与10种浓度的2 nM至40µM的感兴趣化合物在0.2%v/v DMSO中孵育。通过产生7-羟基-3-氰基香豆素、3-[2-(N,N-二乙氨基)乙基]-7-羟基-4-甲基香豆素、7-羟基-4-三氟甲基香豆素或间苯二酚代谢物来测定模型底物的代谢,并通过荧光检测进行测量。在总体积为30µl的384孔微孔板中进行了检测。用由杆状病毒感染的昆虫细胞制备的微粒体孵育45分钟(CYP3A4 BFC测定为20分钟),微粒体含有cDNA衍生的CYP酶,并使用NADPH生成系统。 |
| 细胞实验 |
SK-N-MC CGRP细胞/功能测定[1]
CGRP受体复合物与Gαs类G蛋白偶联。CGRP与这种复合物的结合导致通过Gαs依赖性激活腺苷酸环化酶产生环AMP(腺苷3'5'-环磷酸)。通过测量化合物抑制附着的SK-N-S5 MC细胞中CGRP刺激的环AMP形成的能力,将8评估为人CGRP受体信号传导的抑制剂。SK-N-MC细胞与不同浓度的8或载体预孵育15分钟。加入激动剂(300 pMαCGRP,EC50=26.7+2.7 pM n=3),将样品在室温下孵育30分钟。使用基于HTRF的cAMP检测试剂盒裂解细胞并进行评估。IC50值定义为抑制300pM CGRP刺激的cAMP产生的50%所需的化合物浓度。 渗透性和P-糖蛋白相互作用[1] 通过基于非细胞的平行人工膜渗透性测定(PAMPA)以及基于细胞的Caco-2细胞模型评估8的渗透性。PAMPA渗透性研究使用pION©脂质溶液在室温下在pH 5.5和7.4下用100µM 8进行4小时。这些研究一式三份(即每种化合物3个孔),平均值以nm/秒为单位报告。对培养约21天的单层进行Caco-2细胞研究。在浓度为3.7至100µM、pH 7.4的条件下,对8个样本进行了双向渗透性研究。在P-gp抑制剂(酮康唑20µM和环孢素20µM两侧)存在的情况下,对8(3µM,pH 7.4)进行了随访双向研究。研究了[3H]-地高辛(初始浓度5µM)(P-gp底物)通过Caco-2细胞膜的转运抑制8(初始浓度10µM),以评估该化合物对P-gp的潜在抑制作用。 |
| 动物实验 |
In Vivo Efficacy of 8 [Rimegepant (BMS927711)] in Marmoset Facial Blood Flow[1]
To assess in vivo efficacy and duration of action, a control increase in facial blood flow is induced by administration of hαCGRP (10 µg/kg, IV) 30 min prior (-0.5 hr) to drug delivery. The CGRP antagonist compound under study is administered at time zero (0 min) and three additional hαCGRP challenges are delivered at 45 min intervals for ~2 hr (data collected at 0.25, 1 and 1.75 hrs post-dose). Plasma samples are obtained just before each of the three post-dose hαCGRP administrations, to define the antagonist levels on board at the time of hαCGRP agonist challenge. Each antagonist is administered across a range of doses to define the no-effect, first significant effect and maximal peak effect dose. In the present study, 8 [Rimegepant (BMS927711)] is dosed from 0.3 to 7 mg/kg, SC. The highest dose (7 mg/kg for 8 [Rimegepant (BMS927711)]) represents the maximum dose deliverable within solubility limits and constrained by vehicle volumes that do not disrupt baseline facial blood flow. Following testing, animals are returned to the transport cage and placed on a temperature controlled surface that keeps the animals warm until fully awake and ambulatory. Animals may be tested again after a 14-21 day rest and washout period. In Vivo Methods – Pharmacokinetics in Rat and Cynomolgus Monkey [1] In the rat and monkey studies described below, 8 [Rimegepant (BMS927711)] was administered IV as a solution in a polyethylene glycol 400 (PEG-400)/ethanol (90:10, v/v). Rat Male Sprague-Dawley rats (250-350 g) were used in the PK studies of 8 [Rimegepant (BMS927711)]. Blood samples (~0.3 mL) were collected from the jugular vein into K3EDTA-containing tubes and then centrifuged at 4°C (1500-2000 x g) to obtain plasma, which was stored at -20°C until analysis by LC/MS/MS. In an oral bioavailability study with 8 [Rimegepant (BMS927711)], 2 groups of animals (N = 3 per group) received the compound as an intra-venous (IV) bolus (1 mg/kg) via a jugular vein cannula or as a solution by oral gavage (10 mg/kg to fasted rats). The oral solution contained 8 [Rimegepant (BMS927711)] dissolved in PEG-400/water (90:10, v/v). Serial blood samples were obtained predose and at 0.03 and 0.17 (IV only), 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, and 24 S9 hours post dose. Plasma samples, obtained by centrifugation at 4°C (1500-2000 x g), were stored at -20°C until analysis. Cynomolgus Monkey [1] The PK of 8 was evaluated in a crossover-design study in male cynomolgus monkeys. Following an overnight fast (oral only), 3 animals (5.4 to 6.3 kg) received 8 [Rimegepant (BMS927711)] by IV infusion (1 mg/kg over 5 minutes) via a femoral vein and by oral gavage as either a solution (10 mg/kg dissolved in 79.8% PEG400; 20% N-methylpyrrolidone; 0.2% Tween 80) or suspension of crystalline free base (10 mg/kg suspended in 98.95% water;1% povidone K-30; 0.05% docusate sodium), with at least a 2- week washout between treatments. Serial blood samples (~0.3 mL) were collected from a femoral artery predose and at 0.083, 0.17 (IV only), 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, and 24 hours post dose, and centrifuged at 4°C (1500-2000 x g) to obtain plasma. Samples were stored at -20°C until analysis by LC/MS/MS. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The absolute oral bioavailability of rimegepant is approximately 64%. Following oral administration of the orally disintegrating tablet, maximum plasma concentrations were achieved at 1.5 hours (Tmax). When administered with a high-fat meal, Tmax is delayed by 1 hour, Cmax is decreased by 42-53%, and AUC is decreased by 32-38%. The clinical significance of this difference in pharmacokinetics is unknown. Following oral administration of radiolabeled rimegepant in healthy subjects, 78% of the administered radioactivity was recovered in feces and 24% in urine. Unchanged parent drug was the major component in each, comprising 42% and 51% of the recovered doses, respectively. At steady state, the volume of distribution is approximately 120 L. Metabolism / Metabolites Rimegepant is metabolized by CYP3A4 and, to a lesser extent, CYP2C9. Specific metabolites of rimegepant have not been characterized and no major metabolites have been detected in plasma. Approximately 77% of an administered dose is eliminated unchanged, suggesting metabolism is likely to be a minor means of drug elimination. Biological Half-Life The elimination half-life in healthy subjects is approximately 11 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration controlled trials of rimegepant in several thousand patients, mild-to-moderate serum aminotransferase elevations arose in a small percentage of patients (1% to 2%) and overall rates were not different from those in placebo recipients. In the controlled trials and subsequently with general use, there have been no reports of clinically apparent liver injury attributed to ubrogepant. In contrast, telcagepant, the initial oral CGRP receptor antagonist evaluated as therapy for migraine headaches, was abandoned during development because of several instances of clinically apparent liver injury in recipients that was characterized by marked elevations in serum aminotransferase levels and symptoms of fatigue, nausea and abdominal discomfort arising within 2 to 4 weeks of starting therapy which rapidly resolved with stopping therapy. Similar episodes have not been reported with rimegepant. Likelihood score: E (unlikely cause of clinically apparent acute liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of rimegepant during breastfeeding. However, amounts in breastmilk are low and would not be expected to cause any adverse effects in breastfed infants. If rimegepant is required by the mother of an older infant, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Rimegepant is approximately 96% plasma protein-bound. The specific proteins to which rimegepant binds have not been elucidated. |
| 参考文献 | |
| 其他信息 |
Rimegepant is an oral antagonist of the CGRP receptor developed by Biohaven Pharmaceuticals. It received FDA approval on February 27, 2020 for the acute treatment migraine headache, and was subsequently approved by the European Commission in April 2022 for both the treatment and prevention of migraines. While several parenteral antagonists of CGRP and its receptor have been approved for migraine therapy (e.g. [erenumab], [fremanezumab], [galcanezumab]), rimegepant and [ubrogepant] were the only CGRP antagonists that possessed oral bioavailability until the approval of [atogepant] in 2021. The current standard of migraine therapy involves abortive treatment with "triptans", such as [sumatriptan], but these medications are contraindicated in patients with pre-existing cerebrovascular and cardiovascular disease due to their vasoconstrictive properties. Antagonism of the CGRP pathway has become an attractive target for migraine therapy as, unlike the triptans, oral CGRP antagonists have no observed vasoconstrictive properties and are therefore safer for use in patients with contraindications to standard therapy.
Rimegepant is a Calcitonin Gene-related Peptide Receptor Antagonist. The mechanism of action of rimegepant is as a Calcitonin Gene-related Peptide Receptor Antagonist. Rimegepant is a small molecule inhibitor of the calcitonin gene-related peptide (CGRP) receptor that blocks the action of CGRP, a potent vasodilator believed to play a role in migraine headaches. Rimegepant is approved for treatment of acute migraine attacks. In clinical trials, rimegepant was generally well tolerated with only rare instances of transient serum aminotransferase elevations during therapy and with no reported instances of clinically apparent liver injury. See also: Rimegepant Sulfate (active moiety of). Drug Indication Rimegepant is indicated for the acute treatment of migraine with or without aura in adults. Rimegepant is also indicated for the prevention of episodic migraine in adults. Vydura is indicated for theAcute treatment of migraine with or without aura in adults; Preventative treatment of episodic migraine in adults who have at least 4 migraine attacks per month. Treatment of migraine headaches Prevention of migraine headaches Mechanism of Action The currently accepted theory of migraine pathophysiology considers dysfunction of the central nervous system, in particular the trigeminal ganglion, to be the root cause behind the condition. Activation of the trigeminal ganglion triggers the stimulation of trigeminal afferents that project to the spinal cord and synapse on various pain-sensing intra- and extracranial structures, such as the dura mater. Pain signals are then further transmitted via second-order ascending neurons to the brainstem, hypothalamus, and thalamic nuclei, and from there to several cortical regions (e.g. auditory, visual, motor cortices). The trigeminal ganglion appears to amplify and perpetuate the migraine headache pain through the activation of perivascular fibers and the release of molecules involved in pain generation, such as calcitonin gene-related peptide (CGRP). The α-isoform of CGRP, expressed in primary sensory neurons, is a potent vasodilator and has been implicated in migraine pathogenesis - CGRP levels are acutely elevated during migraine attacks, return to normal following treatment with triptan medications, and intravenous infusions of CGRP have been shown to trigger migraine-like headaches in migraine patients. In addition to its vasodilatory properties, CGRP appears to be a pronociceptive factor that modulates neuronal excitability to facilitate pain responses. Rimegepant is an antagonist of the calcitonin gene-related peptide receptor - it competes with CGRP for occupancy at these receptors, preventing the actions of CGRP and its ability to amplify and perpetuate migraine headache pain, ultimately terminating the headache. Pharmacodynamics Rimegepant helps to abort migraine headaches by preventing the activity of a pronociceptive molecule that has been implicated in migraine pathophysiology. It is intended for use as an abortive migraine therapy and therefore has a relatively rapid onset of effect, with most efficacy trials evaluating for effect at the 2 hour mark. Rimegepant does not require dose adjustment in patients with mild, moderate, or severe renal impairment, nor does it require dose adjustment in patients with mild or moderate hepatic impairment. In clinical trials, plasma concentrations of rimegepant were significantly higher in patients with severe (i.e. Child-Pugh C) hepatic impairment - it should therefore be avoided in this population. Hypersensitivity reactions have occurred during clinical studies and patients should be made aware of this possibility. Rimegepant should be discontinued immediately if hypersensitivity reaction occurs. |
| 分子式 |
C28H28F2N6O3
|
|
|---|---|---|
| 分子量 |
534.57
|
|
| 精确质量 |
534.219
|
|
| 元素分析 |
C, 62.91; H, 5.28; F, 7.11; N, 15.72; O, 8.98
|
|
| CAS号 |
1289023-67-1
|
|
| 相关CAS号 |
1289023-67-1; 1642783-82-1 (0.5 sulfate); 1374024-48-2 (0.5 sulfate 1.5 hydrate); 2377164-85-5 (0.5 sulfate 3 hydrate)
|
|
| PubChem CID |
51049968
|
|
| 外观&性状 |
Solid powder
|
|
| 密度 |
1.5±0.1 g/cm3
|
|
| 折射率 |
1.676
|
|
| LogP |
1.73
|
|
| tPSA |
119.39
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
8
|
|
| 可旋转键数目(RBC) |
4
|
|
| 重原子数目 |
39
|
|
| 分子复杂度/Complexity |
891
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
N[C@H]1[C@H](C2=C(F)C(F)=CC=C2)CC[C@@H](OC(N3CCC(N4C(C=CC=N5)=C5NC4=O)CC3)=O)C6=NC=CC=C61
|
|
| InChi Key |
KRNAOFGYEFKHPB-ANJVHQHFSA-N
|
|
| InChi Code |
InChI=1S/C28H28F2N6O3/c29-20-6-1-4-17(23(20)30)18-8-9-22(25-19(24(18)31)5-2-12-32-25)39-28(38)35-14-10-16(11-15-35)36-21-7-3-13-33-26(21)34-27(36)37/h1-7,12-13,16,18,22,24H,8-11,14-15,31H2,(H,33,34,37)/t18-,22+,24-/m0/s1
|
|
| 化学名 |
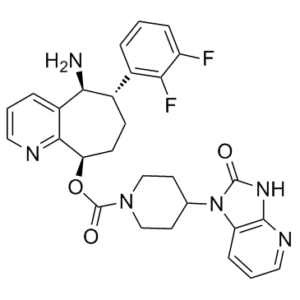
[(5S,6S,9R)-5-amino-6-(2,3-difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyridin-9-yl] 4-(2-oxo-3H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8707 mL | 9.3533 mL | 18.7066 mL | |
| 5 mM | 0.3741 mL | 1.8707 mL | 3.7413 mL | |
| 10 mM | 0.1871 mL | 0.9353 mL | 1.8707 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05207865 | Active Recruiting |
Drug: Rimegepant | Migraine Episodic Migraine Phonophobia |
Pfizer | March 15, 2022 | Phase 4 |
| NCT05399485 | Active Recruiting |
Drug: Rimegepant Drug: Placebo |
Migraine | Pfizer | August 9, 2022 | Phase 3 |
| NCT05399459 | Active Recruiting |
Drug: Rimegepant 25 MG Drug: Rimegepant 75 MG |
Migraine | Pfizer | August 9, 2022 | Phase 3 |
| NCT05371652 | Active Recruiting |
Drug: Rimegepant 75mg Orally Disintegrating Tablets (ODT) |
Acute Migraine | Pfizer | May 19, 2022 | Phase 3 |
| NCT05509400 | Recruiting | Drug: Rimegepant Drug: Placebo |
Migraine | Pfizer | October 18, 2022 | Phase 4 |