| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg | |||
| 50mg | |||
| Other Sizes |
| 靶点 |
N-methyl-D-aspartate (NMDA) receptor subunit 2B (GluN2B) [Ki = 8.1 nM; IC50 = 3.6 nM)
|
|---|---|
| 体外研究 (In Vitro) |
对于激动剂刺激的 NMDA-GluN1a/GluN2BL(tk-) 细胞,rislenemdaz (CERC-301) 可减少钙流入,IC50 为 3.6 nM。当 GluN2B 受体与所有其他靶标(包括 hERG 钾通道)进行比较时,rislenemdaz 表现出至少 1000 倍的选择性。 10 uM 的 rislenemdaz 对 sigma 型受体的作用最小[1]。
体外药理[1] Rislenemdaz/CERC‐301对NMDA‐GluN2B受体的亲和力 [1] CERC‐301有效抑制放射性配体([3H]Compound‐2)与人类NMDA‐GluN1a/GluN2B受体的结合,这些受体在所有被测试物种(大鼠、狗、恒河猴、人)的L(tk‐)细胞和脑组织浆液中表达。利用人颞叶皮层匀浆测定CERC‐301的结合亲和力,在室温和37℃下的K i值分别为3.1 nmol L−1 (0.0031 μmol L−1)和8.1 nmol L−1 (0.0081 μmol L−1)。在其他物种中的发现与人类数据一致(表1)。 Rislenemdaz/CERC‐301对NMDA受体的功能活性和选择性 [1] CERC‐301抑制钙流入激动剂刺激的NMDA‐GluN1a/GluN2B L(tk‐)细胞,IC50为3.6 nmol L−1,但当浓度高达30,000 nmol L−1 (30 μmol L−1)时,对钙流入激动剂刺激的GluN1a/GluN2A细胞没有影响。 Rislenemdaz/CERC‐301对NMDA‐GluN2B受体影响的电生理学研究 [1] CERC - 301的效价通过GluN2B拮抗剂电压箝位法测定。在10 μmol L−1谷氨酸和10 μmol L−1甘氨酸的存在下,测量了CERC‐301的开启率(图2A)和关闭率(图2B)。这些实验表明,k开和k关率分别为1.3 × 105 mol L−1 s−1和~2 × 10−5 s−1,kd约为0.15 nmol L−1。 Rislenemdaz/CERC‐301的反筛选 [1] 当浓度等于或大于10 μmol L−1 (~3584 ng mL−1)时,CERC‐301在酶和放射性配体结合试验中没有表现出显著的活性。CERC‐301对GluN2B受体对所有测试靶标(包括hERG钾通道)表现出至少1000倍的选择性。CERC‐301在10 μmol L−1 (sigma‐1、sigma‐2和非特异性)时对sigma‐型受体也表现出最小的活性。 |
| 体内研究 (In Vivo) |
与赋形剂对照相比,Rislenemdaz (CERC-301)(1、3、10 和 30 mg/kg)显着改善游泳行为(10 mg/kg 时 P<0.05;1、3 和 30 mg/kg 时 P<0.01) )并大大降低了不动的频率(P<0.001)。采样时大鼠血浆中rislenemdaz的水平约为15、120、390、1420、4700和14,110 nM (0.015、0.120、0.390、1.42、4.7和14.11 uM)。这分别相当于约 5%、29%、56%、83%、94% 和 98% RO。大约 0.3 和 0.7 mg/kg,或大约 RO 的 30% 和 50%,分别是增加游泳频率和减少不动的估计 ED50。与载体对照(1 mg/kg P < 0.01;3、10 和 30 mg/kg P < 0.001)的总行驶距离相比,rislenemdaz(1、3、10 和 30 mg/kg)显着增加了距离旅行。很少甚至没有测试[1]。
急性抑郁模型[1] 强迫游泳试验[1] Rislenemdaz(1,3,10和30 mg kg - 1)显著降低不动频率(P < 0.001),显著增加游泳行为(P < 0.01);与车辆对照组相比,10 mg kg - 1的P < 0.05)(图3),但除了3 mg kg - 1的剂量外,不影响攀爬行为(P < 0.05)。与车辆对照组相比,地西帕明(20 mg kg−1)显著降低了静止不动(P < 0.001),显著提高了攀爬行为(P < 0.01),游泳行为没有变化。采样时,CERC‐301的血浆水平分别约为15、120、390、1420、4700和14110 μmol L−1(0.015、0.120、0.390、1.42、4.7和14.11 μmol L−1),对应于大鼠的RO分别约为5、29、56、83、94和98%。游泳频率增加和不活动减少的ED50分别为~0.3和0.7 mg kg - 1,对应的RO为~ 30%和50%。 运动试验[1] Rislenemdaz(1,3,10和30 mg kg - 1)显著增加了旅行距离(P < 0.01);在测试的前5分钟(时间与强迫游泳测试的时间相关),与车辆对照组相比,10和30 mg kg - 1的P < 0.001。CERC‐301(1、3、10和30 mg kg - 1)显著增加总旅行距离(P < 0.01);3、10和30 mg kg−1)与60分钟试验期间的对照相比,P < 0.001。运动活动增加的ED50为~2 mg kg−1,转化为~75%的RO,高于游泳频率增加和不动减少的ED50。0.1和0.3 mg kg - 1剂量组未观察到运动影响(图3)。 遥测大鼠血流动力学研究[1] 单次口服Rislenemdaz/CERC - 301 /CERC - 301,在0.3 ~ 1 mg kg - 1 (ED50≈0.7 mg kg - 1)剂量范围内,清醒大鼠动脉血压呈剂量依赖性短暂升高,且该效应在1 ~ 10 mg kg - 1剂量范围内达到稳定(图4)。在给药后1 - 2小时观察到峰值效应(图4A),与大鼠CERC‐301的PK谱一致。在0.2 mg kg - 1(例如,收缩压+36 mmHg)时,CERC‐301的血流动力学变化幅度小于MK‐801。CERC‐301给药后3.5小时内的血流动力学变化与活动水平呈线性相关(根据遥测记录估计);特别是,活性是HR的预测因子(r2 = 0.67),因此,CERC‐301的兴奋作用可能解释了在大鼠中观察到的HR增加(特别是在高剂量水平下)。该研究还表明α1‐和/或β1‐AR阻断剂可能分别对CERC‐301介导的血压升高(图4B)和心率升高(数据未显示)提供保护。 |
| 酶活实验 |
体外药理测定 [1]
Rislenemdaz/CERC‐301对NMDA‐GluN2B受体的亲和力[1] 如前所述,在室温(或37°C)下进行放射性配体结合试验(Kiss等人,2005;Liverton et al. 2007)。类似的结合实验,如附录S1所述,克隆的人类受体在L(tk‐)细胞中表达,也使用全脑匀浆(大鼠)、额叶皮质匀浆(狗和猕猴)和颞叶皮质匀浆(人)进行。更详细的描述见附录S1。 Rislenemdaz/CERC‐301对NMDA受体的功能活性和选择性 [1] 我们测量了表达GluN1a/GluN2B或GluN1a/GluN2A人受体的L(tk‐)细胞对钙内流的抑制,以确定CERC‐301抑制NMDA受体功能的IC50,如前所述(Kiss et al. 2005;另见附录S1)。 Rislenemdaz/CERC‐301的电生理研究 [1] 利用克隆的人类NMDA‐GluN2B受体进行体外电生理测定,以确定CERC‐301的结合和解离动力学以及效力(Kiss et al. 2005;另见附录S1)。 Rislenemdaz/CERC‐301的反筛谱 [1] CERC‐301在一系列标准受体结合和酶分析中进行评估。CERC - 301在体外以10、30或100 μmol L - 1(分别为~3584、10752或35840 ng mL - 1)检测其与参考配体竞争的能力,以评估可能未检测到的药理活性(研究了bbb150受体和酶,包括sigma型阿片受体)。 非临床药代动力学[1] 血浆蛋白结合[1] 大鼠、狗、恒河猴和人血浆样品(3 mL, N = 3)分别加入2和20 μmol L−1[14℃]CERC‐301,在37℃的摇水浴中孵育30 min。孵育后,采用标准超离心方法测定药物未结合的百分比(Pacifici和Viani 1992)。 |
| 动物实验 |
Neurotoxicity in rats [1]
Four groups of 24 Sprague–Dawley rats (12/sex) were given single doses of vehicle (0.5% methylcellulose [MC] and 0.02% sodium lauryl sulfate [SLS] in deionized water) or Rislenemdaz/CERC‐301 at 10, 30 or 100 mg kg−1 by oral gavage at a dose volume of 10 mL kg−1. An additional group of 12 male rats was given single doses of MK‐801 (a noncompetitive antagonist of the NMDA receptor; positive control) at 10 mg kg−1 by subcutaneous injection at a dose volume of 2 mL kg−1. Six rats per sex in each group were terminated and necropsied at 4 to 6 h postdose, and the remaining rats in each group were terminated and necropsied 3 days postdose (on Day 4). In‐life observations and measurements included body weight and clinical observations. At termination, rats were anesthetized and perfusion fixed. At necropsy, the brain was collected for histopathological evaluation. Animals in Rislenemdaz/CERC‐301 and MK‐801 assessment groups were terminated at the scheduled necropsy intervals (4–6 h postdose or Day 4). All animals were anesthetized with an isoflurane/oxygen mixture and perfused via the left cardiac ventricle with heparinized 0.001% sodium nitrite in saline. The saline wash was followed by perfusion of 10% neutral buffered formalin (NBF). Brains were harvested, weighed, and stored in 10% NBF. The brain was sectioned into 2 mm coronal sections to produce multiple sections in three blocks for each animal. The following brain regions were stained: neocortex, paleocortex, basal nuclei, limbic system, thalamus/hypothalamus, midbrain regions, cerebellum, pons region, and medulla oblongata. All brain sections from all animals sacrificed 4 to 6 h after dosing and all animals sacrificed 3 days after dosing were embedded in paraffin, sectioned at 5 μm, stained with hematoxylin and eosin and examined microscopically. For rats sacrificed on Day 4 (3 days after dosing), serial sections from Blocks 1 and 2 were stained with Fluoro‐Jade B (a stain increasing the sensitivity of evaluating the brain for neuronal degeneration) and glial fibrillary acidic protein (a stain for astrocyte reactions) and examined microscopically. Three additional groups of rats (four males and three females per group) were orally dosed in the same manner with CERC‐301, and 24‐h serial blood samples were obtained and analyzed for Rislenemdaz/CERC‐301 plasma concentrations and evaluated for systemic exposure. In vivo pharmacology [1] Correlation of GluN2B receptor occupancy with plasma drug levels [1] CERC‐301/Rislenemdaz was administered to rats, dogs, and rhesus monkeys, as described in detail in Appendix S1 and previously elsewhere (Liverton et al. 2007). The RO was also determined in rats following IV administration of [3H]Compound‐3. The relationship between plasma concentrations and brain RO was evaluated 15 min after IV and 60 min after oral (PO) dosing of CERC‐301, as described in Appendix S1. Acute depression model [1] Forced swim test [1] Young, adult, male Sprague–Dawley rats were randomly assigned across the treatment groups and were administered vehicle (0.5% MC/0.02% SLS), the reference compound desipramine (20 mg kg−1; a tricyclic antidepressant) dissolved in sterile water, or Rislenemdaz/CERC‐301 (0.1, 0.3, 1, 3, 10, and 30 mg kg−1) suspended in 0.5% MC/0.02% SLS, twice on Day 1 (after habituation; ~24 h prior to test, and prior to dark cycle) and once on Day 2 (30‐min pretest for desipramine and 45‐min pretest for CERC‐301 and vehicle). Each Forced Swim chamber was constructed of clear acrylic (height, 40 cm; diameter, 20.3 cm). Rats were subjected to a predose swim test of one 15‐min session in cylinders containing water at 23°C ± 1°C, followed approximately 24 h later by the experimental 5‐min session. The water level was 16 cm deep during habituation and 30 cm deep during the test. Immobility, climbing, and swimming behaviors were recorded every 5 sec for a total of 60 counts per subject. When an animal was unable to maintain a posture with its nose above water, it was immediately removed from the water and eliminated from the study. Blood was collected at the completion of swim test procedures and plasma was analyzed for Rislenemdaz/CERC‐301 concentrations. Locomotor assay [1] To confirm that the effect of Rislenemdaz/CERC‐301 in the forced swim test was not due to a general increase in activity levels, rats were subjected to a locomotor assay following oral CERC‐301 administration. Adult male Sprague–Dawley rats (N = 42) were randomly assigned across the treatment groups (vehicle or CERC‐301 at 0.1, 0.3, 1, 3, 10, and 30 mg kg−1; N = 6/group). Locomotor activity was assessed during the light cycle in photocell‐monitored cages. Each cage consisted of a standard plastic rat cage (24 × 45.5 cm) surrounded by a stainless steel frame. Infrared photocell beams were located across the long axis of the frame to measure the ambulatory distance traveled. A second set of beams was placed above the floor and was used to measure rearing activity. Photocell beam interruptions were recorded by a computer system. Filter tops were placed on top of the test enclosures during testing. Rats were administered either vehicle or test compound via oral gavage twice on Day 1 (approximately 24 h before the test and prior to dark cycle) and once on Day 2 (45 min prior to placing in the locomotor cages for a 60‐min test). Locomotor activity was captured in 5‐min bins. Hemodynamic effects in telemetered rats [1] To determine the systemic hemodynamic effects, Rislenemdaz/CERC‐301 was administered as a single oral gavage dose to six (n = 6) chronically telemetered rats (implantation at least 7 days prior to the dosing day) at doses of 0.3, 0.6, 1, 3, and 10 mg kg−1 and systemic blood pressure and heart rate values were recorded. The hemodynamic effects were compared to MK‐801 at a dose of 0.2 mg kg−1 given intravenously (in 0.9% saline). In each animal, a single oral gavage dose of Rislenemdaz/CERC‐301 or vehicle (0.5% MC/0.02% SLS) was administered (volume: 5 mL kg−1). A 24‐h recording was performed prior to dosing (vehicle alone) and after each oral dose. In another set of studies, CERC‐301 (1 mg kg−1) was administered in combination with atenolol (β1‐adrenergic blocker, 1 mg kg−1, IV bolus) and prazosin (α1‐adrenergic receptor antagonist, 200 μg kg−1, IV bolus) to elucidate the underlying mechanism of hypertension. Data were analyzed and compared to baseline, with correction for 24‐h predose vehicle control. First‐in‐human pharmacokinetic study [1] The study protocol was approved by the Institutional Review Board and written informed consent was obtained from all subjects. Twenty‐four healthy, young male subjects were assigned to 1 of 3 sequential treatment panels (A, B, and C). For each panel of eight subjects, two subjects received placebo and six subjects were administered single ascending oral doses of Rislenemdaz/CERC‐301 with a minimum 7‐day washout between each dose: Panel A (0.1, 0.2, 0.5, 1, and 2 mg); Panel B (2, 4, 8, and 15 mg, and 4 mg with food); and Panel C (15 and 20 mg). Blood samples were collected pre‐dose and 0.5, 1, 1.5, 2, 3, 4, 6, 9, 12, 18, 24, 30, 48, and 72 h postdose. Plasma samples were analyzed for CERC‐301 concentrations using reversed phase high‐ performance liquid chromatography with tandem mass spectrometric detection. The analytical range was 0.5 to 500 nmol L−1 (0.180 to 180 ng mL−1). |
| 药代性质 (ADME/PK) |
Human pharmacokinetic study [1]
CERC‐301/Rislenemdaz was rapidly absorbed (Fig. 5A) with mean T max within 1 h postdose across all fasted dose levels and terminal elimination half‐life ranging from approximately 12 to 17 h over the 4‐ to 20‐mg dose range. C max and AUC behaved in a dose‐proportional manner over the dose range studied. Dosing in the fed state led to an approximate 1‐h delay in T max and an approximate 56% decrease in C max, but the overall extent of absorption (AUC) was not affected (Fig. 5B, Table 3). Plasma protein binding [1] CERC–301/Rislenemdaz exhibited a high degree of concentration‐independent plasma protein binding at 37°C in rats (89.6%), dogs (97.2%), monkeys (96.9%), and humans (97.7%). |
| 毒性/毒理 (Toxicokinetics/TK) |
Nonclinical safety [1]
Neurotoxicity in rats [1] In rats, single doses of Rislenemdaz/CERC‐301 (10, 30, and 100 mg kg−1) and vehicle control did not produce vacuolation or necrosis in all examined regions of the brain. At these doses, mean C max was approximately 4, 14, and 26 μmol L−1 (1433, 5018, and 9319 ng mL−1), respectively. By contrast, all of the MK‐801 (10 mg kg−1)‐dosed animals had vacuolation and necrosis in cingulate gyrus neurons, consistent with previous reports (Fix et al. 1995). At the 4–6 h time point, the animals treated with MK–801 (six males; Group 5) all had numerous vacuolated neurons in cortical layers 3 and 4 in the cingulate gyrus region of the cerebral cortex. Affected neurons were characterized by numerous, tightly packed, somewhat distinct, vacuoles filling the cytoplasm. On Day 4, all the animals treated with MK‐801 (six males; Group 5) had necrotic neurons (visualized using Fluoro‐Jade B stain) in cortical layers 3 and 4 in the cingulate gyrus region of the cerebral cortex, consistent with previous reports (Fix et al. 1995). In the MK‐801‐treated animals, sections stained (immunohistochemically) for glial fibrillary acidic protein showed a very slight increase in staining in the region of the cingulate gyrus. |
| 参考文献 | |
| 其他信息 |
Mk 0657 has been used in trials studying the treatment of Major Depressive Disorder.
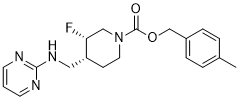
The preclinical pharmacodynamic and pharmacokinetic properties of 4-methylbenzyl (3S, 4R)-3-fluoro-4-[(Pyrimidin-2-ylamino) methyl] piperidine-1-carboxylate (CERC-301/Rislenemdaz), an orally bioavailable selective N-methyl-D-aspartate (NMDA) receptor subunit 2B (GluN2B) antagonist, were characterized to develop a translational approach based on receptor occupancy (RO) to guide CERC-301 dose selection in clinical trials of major depressive disorder. CERC-301 demonstrated high-binding affinity (K i, 8.1 nmol L(-1)) specific to GluN2B with an IC 50 of 3.6 nmol L(-1) and no off-target activity. CERC-301 efficacy was demonstrated in the forced swim test with an efficacy dose (ED 50) of 0.3-0.7 mg kg(-1) (RO, 30-50%); increase in locomotor activity was observed at ED 50 of 2 mg kg(-1), corresponding to an RO of 75%. The predicted 50% RO concentration (Occ50) in humans was 400 nmol L(-1), similar to that predicted for rat, dog, and monkey (300, 200, and 400 nmol L(-1), respectively). Safety pharmacology and neurotoxicity studies raised no specific safety concerns. A first-in-human study in healthy males demonstrated a dose-proportional pharmacokinetic profile, with T max of ~1 h and t 1/2 of 12-17 h. Based on the preclinical and pharmacodynamic data, doses of ≥8 mg in humans are hypothesized to have an acceptable safety profile and result in clinically relevant peak plasma exposure. [1] The studies in conscious telemetered rats demonstrates that Rislenemdaz/CERC‐301, when given orally, increased arterial blood pressure transiently, and in a dose‐dependent manner between 0.3 and 1 mg kg−1, and this effect plateaued at 1–10 mg kg−1. Interestingly, the ED50 for blood pressure effects was similar to ED50 for the forced swim test. The magnitude of change in hemodynamics with CERC‐301 was less than that of MK‐801, a broad NMDA receptor antagonist. The findings with MK‐801 were consistent with previously published mechanistic data on MK‐801 (Lewis et al. 1989). The changes in HR and blood pressure may be partially explained by drug‐dependent enhanced movement of rats; dose‐dependent movement analysis was also observed in the Locomotor study, and demonstrate a supporting trend at these higher doses. This study also demonstrated that, α1‐adernergic blockade may provide protection from CERC‐301 mediated increases in blood pressure; however, more studies are required to further elucidate the underlying mechanisms. [1] Unlike some clinically utilized NMDA receptor antagonists (e.g., ketamine or memantine), CERC‐301 showed no evidence of neurotoxicity in rats given single oral doses at up to 100 mg kg−1. This result is consistent with those obtained with other GluN2B‐specific receptor antagonists (e.g., CP‐101,606 and Ro 63‐1908), which have demonstrated a lack of neurotoxicity, and in the case of CP‐101,606 paradoxically may have neuroprotective potential in the developing brain (Gill et al. 2002; Lewis et al. 2012). Lack of a nonclinical neurotoxicity signal with GluN2B‐specific antagonists, along with the absence of ketamine‐like psychotomimetic effects in the clinical setting allows administration of these agents at higher doses to achieve higher receptor occupancies (than what has been predicted herein), should the clinical data necessitate that in the future clinical trials. [1] Pharmacokinetic data from the first‐in‐human study with CERC‐301 demonstrated mean C max ranging from 0.007 μmol L−1 (2.4 ng mL−1) to 1.65 μmol L−1 (590.3 ng mL−1) across the 0.1 to 20 mg single dose (fasted) range, with a half‐life that makes the drug suitable for once‐daily dosing in human subjects. [1] CERC‐301 is a potent orally bioavailable, selective NMDA‐GluN2B receptor antagonist with antidepressant effects. Based on the preclinical PK and pharmacodynamic data, chronic 8‐mg daily administration in humans is hypothesized to have an acceptable safety profile, result in clinically relevant plasma and brain concentrations, and exhibit rapid onset of antidepressant activity. [1] |
| 分子式 |
C19H23FN4O2
|
|---|---|
| 分子量 |
358.409927606583
|
| 精确质量 |
358.18
|
| 元素分析 |
C, 63.67; H, 6.47; F, 5.30; N, 15.63; O, 8.93
|
| CAS号 |
808732-98-1
|
| 相关CAS号 |
808733-06-4 (HCl);808732-98-1;1893392-76-1 (mesylate);
|
| PubChem CID |
11394238
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
527.4±60.0 °C at 760 mmHg
|
| 闪点 |
272.7±32.9 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.584
|
| LogP |
2.73
|
| tPSA |
67.4
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
26
|
| 分子复杂度/Complexity |
439
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC1=CC=C(C=C1)COC(=O)N2CC[C@@H]([C@@H](C2)F)CNC3=NC=CC=N3
|
| InChi Key |
RECBFDWSXWAXHY-IAGOWNOFSA-N
|
| InChi Code |
InChI=1S/C19H23FN4O2/c1-14-3-5-15(6-4-14)13-26-19(25)24-10-7-16(17(20)12-24)11-23-18-21-8-2-9-22-18/h2-6,8-9,16-17H,7,10-13H2,1H3,(H,21,22,23)/t16-,17-/m1/s1
|
| 化学名 |
4-methylbenzyl (3S,4R)-3-fluoro-4-((pyrimidin-2-ylamino)methyl)piperidine-1-carboxylate
|
| 别名 |
CERC-301; CERC301; Rislenemdaz; 808732-98-1; Rislenemdaz [USAN]; Rislenemdaz, (-)-; MK-0657, (-)-; 4-Methylbenzyl (3S,4R)-3-fluoro-4-((pyrimidin-2-ylamino)methyl)piperidine-1-carboxylate; CERC 301; MK-0657; MK 0657; MK0657;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~279.01 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7901 mL | 13.9505 mL | 27.9010 mL | |
| 5 mM | 0.5580 mL | 2.7901 mL | 5.5802 mL | |
| 10 mM | 0.2790 mL | 1.3951 mL | 2.7901 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
(A) Time course ofN‐methyl‐D‐aspartate (NMDA) receptor inhibition by CERC‐301 at three concentrations of 30, 100, and 300nmolL−1.Pharmacol Res Perspect. 2015 Dec; 3(6): e00198. |
|---|
In‐vivo efficacy and potential central nervous system (CNS) side effects of CERC‐301 when orally administered in rats. Efficacy is depicted by a reduction in immobility frequency (filled circles; left axis) during the forced swim test. Potential CNS side effect is depicted by an increase in total distance traveled (open squares; right axis) as a function of dose.Pharmacol Res Perspect. 2015 Dec; 3(6): e00198. |
Effects of a single oral dose ofCERC‐301 on systolic blood pressure.Pharmacol Res Perspect. 2015 Dec; 3(6): e00198. |