| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|
| 靶点 |
TSPO/mitochondrial translocator protein
|
|---|---|
| 体外研究 (In Vitro) |
在 SHSY-5Y 神经母细胞瘤细胞中,4'-氯地西泮具有针对淀粉样蛋白-β (Aβ) 的神经保护特性。在器官型海马制剂中,Aβ 会降低细胞活力,但剂量为 100nM 和 1000nM 的 4'-氯地西泮具有神经保护作用。超氧化物歧化酶 (SOD) 表达的上调与 4'-氯地西泮对 Aβ 的神经保护作用有关 [1]。在葡萄糖剥夺的细胞中,4'-氯扎西泮可减少核碎裂并维持细胞活力。在用 4'-氯地西泮处理的细胞中,这些效应伴随着自由基形成的减少和线粒体功能的保存 [2]。
|
| 细胞实验 |
转运蛋白(TSPO)是一种线粒体外膜蛋白,参与胆固醇向线粒体的转运,这是合成类固醇激素的第一步,也是调节线粒体通透性转换孔打开和凋亡的第一步。研究表明,TSPO的激活可能会促进神经退行性变和脑损伤实验模型中的神经保护作用。在之前的一项研究中,我们小组表明,TSPO配体4'-氯二氮卓(4'-CD)对SHSY-5Y神经母细胞瘤细胞中的淀粉样蛋白β(aβ)具有神经保护作用。本研究旨在评估4'-CD是否也对器官型海马培养物中的Aβ具有神经保护作用,并确定其作用机制。Aβ降低了器官型海马培养物的细胞活力,而4'-CD在100nM和1000nM的剂量下具有神经保护作用。4'-CD对Aβ的神经保护作用与超氧化物歧化酶(SOD)表达的增加有关。过氧化氢酶、胶质纤维酸性蛋白、Akt和前天冬氨酸蛋白酶-3的表达没有差异。总之,我们的研究结果表明,4'-CD通过调节SOD蛋白表达的机制对Aβ具有神经保护作用[1]。
转运蛋白(TSPO),以前称为外周型苯二氮卓受体(PBR),被认为是类固醇生成的重要调节因子,也是神经系统疾病的潜在治疗靶点。先前的证据表明,TSPO配体可以在损伤过程中保护细胞,防止中枢神经系统(CNS)细胞凋亡。然而,其在代谢损伤下对星形胶质细胞的作用尚不清楚。在这项研究中,我们探讨了TSPO配体4'-氯二氮卓(Ro5-4864)是否可以在葡萄糖剥夺下保护星形胶质细胞线粒体。我们的结果表明,4'-氯地西泮保留了细胞活力,并减少了葡萄糖缺乏细胞的核碎裂。这些影响伴随着用4'-氯地西泮处理的细胞中自由基的产生减少和线粒体功能的维持。最后,我们的研究结果表明,TSPO可能通过保护暴露于葡萄糖戒断的星形胶质细胞中的线粒体功能来参与减少氧化应激[2]。 |
| 参考文献 |
[1]. B D Arbo, et al. 4'-Chlorodiazepam is neuroprotective against amyloid-beta in organotypic hippocampal cultures. J Steroid Biochem Mol Biol. 2017 Jul;171:281-287.
[2]. Eliana Baez, et al. 4'-Chlorodiazepam Protects Mitochondria in T98G Astrocyte Cell Line from Glucose Deprivation. Neurotox Res. 2017 Aug;32(2):163-171. |
| 其他信息 |
Mechanism of Action
Mitochondria isolated from rat brain were found to cleave cholesterol to produce pregnenolone, the precursor for hormonal steroids, at a mean rate of 21.0 pmol pregnenolone.mg protein-1.min-1. This rate-limiting step in steroidogenesis was significantly stimulated by PK 11195 (1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3-isoquinoline carboxamide) and Ro5 4864 (4'-chlorodiazepam), ligands which bind to peripheral benzodiazepine receptors with high affinity. Low-affinity ligands for the peripheral benzodiazepine receptor such as Ro15 1788 (ethyl-8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5 alpha][1,4] benzo-3-carboxylate) and clonazepam had no significant effect on the rate of pregnenolone synthesis. Furthermore, the rank order of potency of these compounds as inhibitors of [3H]Ro5 4864 binding was identical to the rank order for steroid production. Since the 86-amino acid peptide diazepam binding inhibitor is also thought to bind to the peripheral benzodiazepine receptor, four fragments of this peptide, a random sequence and steroidogenesis activator peptide were also evaluated for their ability to interact with peripheral benzodiazepine receptors and to stimulate steroidogenesis in rat brain mitochondria. Steroidogenesis activator peptide and two fragments of diazepam binding inhibitor significantly stimulated pregnenolone biosynthesis. In contrast to the peripheral benzodiazepine receptor ligands, no correlation between peptide potency in displacing [3H]Ro5 4864 binding and steroidogenesis was observed. Peripheral benzodiazepine receptors mediate cholesterol translocation between the outer and inner mitochondrial membranes in steroidogenic tissues. They are found in many other tissues too, including liver. We studied the effect of the peripheral benzodiazepine receptor ligands PK11195 [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoquinoline-3-carboxa mid e], Ro 5-4864 (4-chlorodiazepam), hemin, protoporphyrin IX and N-methyl protoporphyrin IX on cholesterol mitochondrial intermembrane transport of cholesterol in vitro in rat liver. Endogenous cholesterol translocation from outer to inner mitochondrial membranes was significantly increased by PK11195 and N-methyl protoporphyrin IX (140% and 150% increase, respectively, at 1 microM, P<0.01). 5 microM protoporphyrin IX, 1 microM Ro 5-4864 and 5 microM hemin was ineffective. When mitochondria were labeled with exogenous [4-14C]cholesterol, PK11195 and N-methyl protoporphyrin IX were the most effective in increasing total cholesterol incorporation and cholesterol translocation into inner membranes, and their effect was dose-dependent. These data suggest that in liver the binding to peripheral benzodiazepine receptors is related to cholesterol translocation and the interaction of ligands with these receptors may play a role in the complex mechanism of regulation of cholesterol traffic between liver mitochondrial membranes. Effects of various benzodiazepines were investigated in ovariectomized rat isolated uterus which had been chronically pre-treated with different female sex hormones: oestrogen, progesterone and oestrogen + progesterone. Uteri obtained from all groups developed a spontaneous, rhythmic activity. The spontaneous activity observed in control uterus was either inhibited in a concentration-dependent manner by diazepam, 4'-chlorodiazepam, clonazepam or 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxam ide (PK 11195), or was abolished in [Ca2+]o-free solution. Diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 all caused a concentration-dependent relaxation of the [K+]o-pre-contracted uterus with the relative order of potency: PK 11195 > 4'-chlorodiazepam > diazepam > clonazepam. Administration of [Ca2+]o (1 microM to 10 mM) caused a concentration-dependent contraction of uterus, bathed in [Ca2+]o-free physiological salt solution obtained from different pre-treatment groups. Incubation with different concentrations (uM) of diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 caused a decrease in response to [Ca2+]o-induced contraction in all groups of rat uteri. These results indicate that micromolar benzodiazepine binding sites exist in rat uterus. Diazepam, 4'-chlorodiazepam, clonazepam and PK 11195 caused relaxation of pre-contracted rat uterus and this effect may involve the inhibition of influx of [Ca2+]o and the relaxing effects of different benzodiazepines observed in this study can be modulated by pre-treatment with different female hormones. The interactions of the atypical benzodiazepine 4'-chlorodiazepam (Ro 5-4864) with functionally expressed human GABAA receptor cDNAs were determined. Cotransfection of human alpha 2, beta 1, and gamma 2 subunits was capable of reconstituting a 4'-chlorodiazepam recognition site as revealed by a dose-dependent potentiation of t-[35S]butylbicyclophosphorothionate ([35S]-TBPS) binding to the GABA-activated chloride channel. This site is found on GABAA receptor complexes containing sites for GABA agonist-like benzodiazepines and neuroactive steroids. The importance of the alpha subunit was further demonstrated as substitution of either alpha 1 or alpha 3 for the alpha 2 subunit did not reconstitute a 4'-chlorodiazepam recognition site that was capable of modulating [35S]TBPS binding under the same experimental conditions. The 4'-chlorodiazepam modulatory site was shown to be distinct from the benzodiazepine site, but the phenylquinolines PK 8165 and PK 9084 produced effects similar to 4'-chlorodiazepam, consistent with the previous analysis of the 4'-chlorodiazepam site in brain homogenates. Further analysis of the subunit requirements revealed that coexpression of alpha 2 and beta 1 alone reconstituted a 4'-chlorodiazepam recognition site. It is interesting, however, that the 4'-chlorodiazepam site was found to inhibit [35S]TBPS binding to the GABA-activated chloride channel. Thus, the 4'-chlorodiazepam site may be reconstituted with only the alpha and beta polypeptides. For more Mechanism of Action (Complete) data for 4-CHLORODIAZEPAM (6 total), please visit the HSDB record page. |
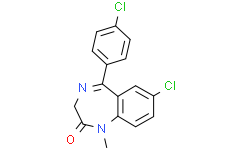
| 分子式 |
C16H12CL2N2O
|
|---|---|
| 分子量 |
319.185
|
| 精确质量 |
318.033
|
| 元素分析 |
C, 60.21; H, 3.79; Cl, 22.21; N, 8.78; O, 5.01
|
| CAS号 |
14439-61-3
|
| PubChem CID |
1688
|
| 外观&性状 |
Typically exists as white to light yellow solids at room temperature
|
| 密度 |
1.35g/cm3
|
| 沸点 |
517.1ºC at 760mmHg
|
| 熔点 |
160-163ºC
|
| 闪点 |
266.6ºC
|
| 蒸汽压 |
8.43E-11mmHg at 25°C
|
| 折射率 |
1.647
|
| LogP |
3.307
|
| tPSA |
32.67
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
21
|
| 分子复杂度/Complexity |
432
|
| 定义原子立体中心数目 |
0
|
| SMILES |
ClC1C([H])=C([H])C2=C(C=1[H])C(C1C([H])=C([H])C(=C([H])C=1[H])Cl)=NC([H])([H])C(N2C([H])([H])[H])=O
|
| InChi Key |
PUMYFTJOWAJIKF-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H12Cl2N2O/c1-20-14-7-6-12(18)8-13(14)16(19-9-15(20)21)10-2-4-11(17)5-3-10/h2-8H,9H2,1H3
|
| 化学名 |
7-chloro-5-(4-chlorophenyl)-1-methyl-3H-1,4-benzodiazepin-2-one
|
| 别名 |
RO-5-4864; RO 5-4864; Chlordiazepam; RO5-4864; 4-Chlorodiazepam; Ro 5-4864; 2H-1,4-Benzodiazepin-2-one, 7-chloro-5-(4-chlorophenyl)-1,3-dihydro-1-methyl-; Ro-05-4864; RO5-4864
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~313.29 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.83 mM) (饱和度未知) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1329 mL | 15.6647 mL | 31.3293 mL | |
| 5 mM | 0.6266 mL | 3.1329 mL | 6.2659 mL | |
| 10 mM | 0.3133 mL | 1.5665 mL | 3.1329 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。