| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
D3 Receptor ( Ki = 0.71 nM ); D2 Receptor ( Ki = 4-15 nM ); D5 Receptor ( Ki = 4-15 nM ); D4 Receptor ( Ki = 4-15 nM ); D1 Receptor ( Ki = 83 nM ); 5-HT1A Receptor ( Ki = 30 nM ); 5-HT7 Receptor ( Ki = 86 nM )
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:罗替高汀是多巴胺 D2 和 D3 受体激动剂。 D2 和 D3 的 Ki 值分别为 13 和 0.71 nM。罗替高汀还对 5-HT1A 和肾上腺素能 α2B 受体具有显着的亲和力。罗替高汀具有抗帕金森病活性。激酶测定:结合测定在 96 孔聚丙烯管中进行,D1 和 D4 膜的终体积为 2 mL,D2、D3 和 D5 膜的终体积为 1 mL,包含:50 μL 放射性配体、10 μL 药物/缓冲液/非特异性结合、缓冲液(终浓度 50 mM Tris-HCl pH 7.4、MgCl2 2 mM)和膜(D2 和 D3 为 5 μg 蛋白,D1 和 D5 为 25 μg 蛋白)。在 25°C 孵育 120 分钟后,通过预先浸泡在 0.1% 聚乙烯亚胺中的 A/C 玻璃纤维过滤器进行快速真空过滤,测定结合的放射性配体。用 2 mL 冰冷的抛光缓冲液(Tris-HCl 50 mM,pH 7.4,4°C)洗涤过滤器四次,并通过液体闪烁计数测定保留的放射性。
|
||
| 体内研究 (In Vivo) |
在已启动的大鼠中,罗替高汀(N-0437;N-0923;0.035、0.1 和 0.35 mg/kg)以剂量依赖性方式诱导对侧转向行为。在未接受药物治疗的大鼠中,单独使用罗替戈汀或与 SCH 39166 联合使用时,与初次给药的大鼠相比,罗替高汀诱导的转向行为有所减少。
|
||
| 酶活实验 |
在 96 孔聚丙烯管中,D1 和 D4 膜的终体积为 2 mL,D2、D3 和 D5 膜的终体积为 1 mL,进行结合测定。这些管含有以下材料:50 μL 放射性配体、10 μL 药物/缓冲液/非特异性结合、缓冲液(终浓度 50 mM Tris-HCl pH 7.4、MgCl2 2 mM)和膜(5 μg 蛋白质用于 D2 和 D3, D1 和 D5 为 25 μg 蛋白质)。通过预先浸泡在 0.1% 聚乙烯亚胺中的 A/C 玻璃纤维过滤器进行快速真空过滤,用于在 25°C 孵育 120 分钟后测定结合的放射性配体。液体闪烁计数用于确定过滤器用 2 mL 冰冷抛光缓冲液(Tris-HCl 50 mM,pH 7.4,4°C)清洗四次后保留的放射性。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability varies depending on the application site. Differences in bioavailability were very small between the abdomen and hip (<1%). In contrast, the shoulder and thigh had a very large different in measured bioavailability (46%), with the shoulder showing the higher value. Tmax, 8 mg dose = 15 - 18 hours (it take approximately 3 hours until rotigotine reaches detectable levels in the plasma). The peak concentration cannot be observered. Steady state is reached in 2-3 days. Urine (71%), Fecal (23%). Most of rotigotine that is excreted in the urine is in the form of inactive conjugates. Unchanged drug made up less <1%. The weight normalized apparent volume of distribution, (Vd/F), in humans is approximately 84 L/kg after repeated dose administration. Results obtained with the patch administration in animals showed that the silicone based patch was superior to the acrylic based patch with respect to substance release. Following repeated dosing, 81 and 93 % substance was released from the silicone patch on the rat and monkey, respectively. The corresponding % release from the acrylic based patch was 28 and 22 %, respectively. The weight normalized apparent volume of distribution (Vd/F) in humans is approximately 84 L/kg after repeated dose administration. The binding of rotigotine to human plasma proteins is approximately 92% in vitro and 89.5% in vivo. When single doses of 8 mg/24 hours are applied to the trunk, there is an average lag time of approximately 3 hours until drug is detected in plasma (range 1 to 8 hours). Tmax typically occurs between 15 to 18 hours post dose but can occur from 4 to 27 hours post dose. However, there is no characteristic peak concentration observed. Rotigotine displays dose-proportionality over a daily dose range of 1 mg/24 hours to 24 mg/24 hours. In the clinical studies of rotigotine effectiveness, the transdermal system application site was rotated from day to day (abdomen, thigh, hip, flank, shoulder, or upper arm) and the mean measured plasma concentrations of rotigotine were stable over the 6 months of maintenance treatment. Relative bioavailability for the different application sites at steady-state was evaluated in subjects with Parkinson's disease. In a single trial conducted in patients with early-stage Parkinson's disease, differences in bioavailability ranged from less than 1% (abdomen vs. hip) to 46% (shoulder vs. thigh) with shoulder application showing higher bioavailability. Rotigotine is primarily excreted in urine (approximately 71%) as inactive conjugates of the parent compound and N-desalkyl metabolites. A smaller proportion is excreted in feces (approximately 23%). The major metabolites found in urine were rotigotine sulfate (16% to 22% of the absorbed dose), rotigotine glucuronide (11% to 15%), and N-despropyl-rotigotine sulfate metabolite (14% to 20%) and N-desthienylethyl-rotigotine sulfate metabolite (10% to 21%). Approximately 11% is renally eliminated as other metabolites. A small amount of unconjugated rotigotine is renally eliminated (less than 1% of the absorbed dose). For more Absorption, Distribution and Excretion (Complete) data for ROTIGOTINE (9 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic (CYP-mediated). Rotigotine is extensively and rapidly metabolized by conjugation and N-dealkylation. After intravenous dosing the predominant metabolites in human plasma are sulfate conjugates of rotigotine, glucuronide conjugates of rotigotine, sulfate conjugates of the N-despropyl-rotigotine and conjugates of N-desthienylethyl-rotigotine. Multiple CYP isoenzymes, sulfotransferases and two UDP-glucuronosyltransferases catalyze the metabolism of rotigotine. CYP2C19 was found to be the major CYP isoform involved in the phase 1 metabolism of rotigotine. However, multiple CYP-isoforms appear to be capable of catalyzing the metabolism. In vitro studies suggest a low risk for drug-drug interactions with co-administered drugs which are substrates of CYP isoforms in vivo. Also, no induction of human liver CYP isoforms has been found. No potential for displacement of rotigotine by warfarin and vice versa was detected with human serum albumin in vitro. Rotigotine was found not to be a substrate for P-glycoprotein and does not modulate digoxin transport in vitro. Following absorption rotigotine was rapidly metabolised. Three phase 1 metabolites showed pharmacological activity. However, pharmacokinetics of these metabolites were not required as their presence in plasma was too low. The major metabolite observed in animal hepatocytes, the glucuronide conjugate of rotigotine, was in vivo excreted into bile and only reached the blood system at low levels. Conjugates of the Ndealkylated metabolites were found to be the major metabolites in plasma. Following subcutaneous administration, the sulfate and the glucuronide conjugates of the SPM 9206 metabolite and the sulfate conjugates of the SPM 9257 and the sulfate of the desthienylethyl despropyl metabolite were found to be the major metabolites in plasma. In human plasma, the sulphate conjugates of rotigotine, the SPM 9206 and the SPM 9257 metabolite were found to be the major metabolites. All the human major metabolites found in plasma were also found in the plasma of the main toxicological species. Rotigotine is extensively metabolized by conjugation and N-dealkylation. After intravenous dosing the predominant metabolites in human plasma are sulfate conjugates of rotigotine, glucuronide conjugates of rotigotine, sulfate conjugates of the N-despropyl-rotigotine and conjugates of N-desthienylethyl-rotigotine. Multiple CYP isoenzymes, sulfotransferases and two UDP-glucuronosyltransferases catalyze the metabolism of rotigotine. Rotigotine is primarily excreted in urine (approximately 71%) as inactive conjugates of the parent compound and N-desalkyl metabolites. A smaller proportion is excreted in feces (approximately 23%). The major metabolites found in urine were rotigotine sulfate (16% to 22% of the absorbed dose), rotigotine glucuronide (11% to 15%), and N-despropyl-rotigotine sulfate metabolite (14% to 20%) and N-desthienylethyl-rotigotine sulfate metabolite (10% to 21%). Approximately 11% is renally eliminated as other metabolites. A small amount of unconjugated rotigotine is renally eliminated (less than 1% of the absorbed dose). Biological Half-Life After removal of the patch, plasma levels decreased with a terminal half-life of 5 to 7 hours. The pharmacokinetic profile showed a biphasic elimination with an initial half-life of 3 hours. After removal of the patch, plasma levels decreased with a terminal half-life of 5 to 7 hours. The pharmacokinetic profile showed a biphasic elimination with an initial half-life of 3 hours. ... A single transdermal patch delivering 2 mg/24 hr rotigotine (patch content 4.5 mg) was applied to the ventral/lateral abdomen for 24 hr. ... The pharmacokinetic analysis included 48 subjects (24 Japanese, 24 Caucasian). ... The terminal half-life for unconjugated rotigotine was 5.3 hr in Japanese subjects and 5.7 hr in Caucasians; corresponding values for total rotigotine were 8.6 hr and 9.6 hr. ... |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Rotigotine is a white to off white powder formulated into a transdermal system (patch). It is a nonergot-derivative dopamine receptor agonist used for the symptomatic management of idiopathic parkinsonian syndrome. It is also used for the symptomatic management of moderate-to-severe primary restless legs syndrome. HUMAN EXPOSURE AND TOXICITY: The most likely symptoms of overdose would be those related to the pharmacodynamic profile of a dopamine agonist, including nausea, vomiting, hypotension, involuntary movements, hallucinations, confusion, convulsions, and other signs of excessive dopaminergic stimulation. Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic behavior during rotigotine treatment or after starting or increasing the dose of rotigotine. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior may consist of one or more of the following: paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium. These various manifestations of psychotic behavior were also observed during the clinical development of rotigotine for early- and advanced-stage Parkinson's disease and Restless Legs Syndrome. Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including rotigotine, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. ANIMAL STUDIES: Two-year carcinogenicity studies of rotigotine were conducted in mice at doses of 0, 3, 10, and 30 mg/kg and in rats at doses of 0, 0.3, 1, and 3 mg/kg; in both studies rotigotine was administered subcutaneously once every 48 hours. No significant increases in tumors occurred in mice at doses up to 9 times the maximum recommended human dose (MRHD) in Parkinson's disease (8 mg/24 hours). In rats, there were increases in Leydig cell tumors and in uterine tumors (adenocarcinomas, squamous cell carcinomas) at all doses. The endocrine mechanisms believed to be involved in the production of these tumors in rats are not considered relevant to humans. Therefore, there were no tumor findings considered relevant to humans at plasma exposures (AUC) up to 4 to 6 times that in humans at the MRHD. Rotigotine administered subcutaneously (10, 30, or 90 mg/kg/day) to pregnant mice during organogenesis resulted in increased incidences of delayed skeletal ossification and decreased fetal body weights at the two highest doses and an increase in embryo-fetal death at the high dose. Rotigotine administered subcutaneously (0.5, 1.5, or 5 mg/kg/day) to pregnant rats during organogenesis resulted in increased embryo-fetal death at all doses. When rotigotine was administered subcutaneously (5, 10, or 30 mg/kg/day) to pregnant rabbits during organogenesis, an increase in embryo-fetal death occurred at the two highest doses tested. In a study in which rotigotine was administered subcutaneously (0.1, 0.3, or 1 mg/kg/day) to rats throughout pregnancy and lactation, impaired growth and development during lactation and long-term neurobehavioral abnormalities were observed in the offspring at the highest dose tested; when those offspring were mated, growth and survival of the next generation were adversely affected. When rotigotine was administered subcutaneously (1.5, 5, or 15 mg/kg/day) to female rats prior to and during mating and continuing through gestation day 7, an absence of implantation was observed at all doses. In male rats treated from 70 days prior to and during mating, there was no effect on fertility; however, a decrease in epididymal sperm motility was observed at the highest dose tested. When rotigotine was administered subcutaneously to female mice at doses of 10, 30, and 90 mg/kg/day from 2 weeks until 4 days before mating and then at a dose of 6 mg/kg/day (all groups) from 3 days before mating until gestation day 7, a markedly reduced (low dose) or complete absence of implantation (mid and high doses) was observed. The effects on implantation in rodents are thought to be due to the prolactin-lowering effect of rotigotine. In humans, chorionic gonadotropin, not prolactin, is essential for implantation. Rotigotine was negative in the in vitro bacterial reverse mutation (Ames) and in the in vivo micronucleus assays. Rotigotine was mutagenic and clastogenic in the in vivo mouse lymphoma tk assay Hepatotoxicity In multiple, controlled trials in Parkinson disease and restless leg syndrome, rotigotine transdermal patches were not associated with serum enzyme elevations, liver related severe adverse events or instances of clinically apparent liver injury. Since the approval and more wide scale use of rotigotine, there have been no published case reports of liver injury associated with its use and hepatotoxicity is not mentioned in the product label. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of rotigotine during breastfeeding, but it suppresses serum prolactin and may interfere with breastfeeding. An alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information in nursing mothers was not found as of the revision date. Rotigotine lowers serum prolactin. The prolactin level in a mother with established lactation may not affect her ability to breastfeed. Protein Binding 92% in vitro and 89.5% in vivo. Interactions Concurrent oral administration of levodopa/carbidopa (100/25 mg twice daily) and transdermal rotigotine (4 mg/24 hours) in patients with restless legs syndrome had no effect on the steady-state pharmacokinetics of any of the drugs. Transdermal rotigotine may potentiate the therapeutic effects of levodopa as well as its adverse dopaminergic effects (including dyskinesia). Concurrent administration of transdermal rotigotine (3 mg/24 hours) did not substantially affect the pharmacodynamics or pharmacokinetics of an oral estrogen-progestin combination contraceptive (ethinyl estradiol 0.03 mg with 0.15 mg levonorgestrel) in healthy females. Possible interactions with rotigotine and other forms of hormonal contraceptives have not been evaluated to date. Dopamine antagonists (e.g., antipsychotic agents, metoclopramide) may diminish the effectiveness of rotigotine. Transdermal rotigotine may potentiate the therapeutic and/or adverse dopaminergic effects (including dyskinesia) of other dopamine agonists used in the treatment of parkinsonian syndrome and restless legs syndrome. Alcohol and other CNS depressants (e.g., sedatives, anxiolytics, antidepressants, antipsychotics, opiate analgesics) may increase the risk of additive effects, including somnolence and falling asleep during activities of daily living, in patients receiving transdermal rotigotine, and should therefore be used concomitantly with caution. |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Dopamine Agonists /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health(NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Rotigotine is included in the database. Neupro is indicated for the treatment of Parkinson's disease. /Included in US product label/ Neupro is indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome. /Included in US product label/ For more Therapeutic Uses (Complete) data for ROTIGOTINE (6 total), please visit the HSDB record page. Drug Warnings Post-marketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic behavior during Neupro treatment or after starting or increasing the dose of Neupro. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior may consist of one or more of the following: paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium. These various manifestations of psychotic behavior were also observed during the clinical development of Neupro for early- and advanced-stage Parkinson's disease and Restless Legs Syndrome. There was an increased risk for hallucinations in patients with advanced-stage Parkinson's disease treated with Neupro. In patients taking the maximum recommended Neupro dose, the incidence of hallucinations was 7% for Neupro and 3% for placebo, and this treatment difference increased with increasing dose. Hallucinations were of sufficient severity to cause discontinuation of treatment (mainly during the dose escalation/titration period) in 3% of advanced-stage Parkinson's disease patients treated with the maximum recommended dose of Neupro compared with 1% of placebo-treated patients. Hallucinations have also been reported in post-marketing reports. Patients with a major psychotic disorder should ordinarily not be treated with Neupro because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of Neupro. Patients may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more of the medications, including Neupro, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with Neupro. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking Neupro. For more Drug Warnings (Complete) data for ROTIGOTINE (26 total), please visit the HSDB record page. Pharmacodynamics Rotigotine is an agonist at all 5 dopamine receptor subtypes (D1-D5) but binds to the D3 receptor with the highest affinity. It is also an antagonist at α-2-adrenergic receptors and an agonist at the 5HT1A receptors. Rotigotine also inhibits dopamine uptake and prolactin secretion. There is no indication of a QT/QTc prolonging effect of Neupro in doses up to 24 mg/24 hours. The effects of Neupro at doses up to 24 mg/24 hours (supratherapeutic doses) on the QT/QTc interval was evaluated in a double-blind, randomized, placebo- and positive-controlled (moxifloxacin 400 mg IV, single dose) parallel-group trial with an overall treatment period of 52 days in male and female patients with advanced-stage Parkinson's disease. Assay sensitivity was confirmed by significant QTc prolongation by moxifloxacin. |
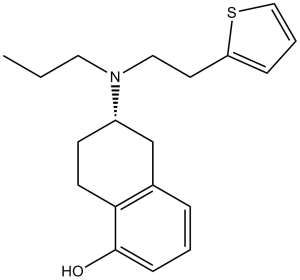
| 分子式 |
C19H25NOS
|
|
|---|---|---|
| 分子量 |
315.47
|
|
| 精确质量 |
315.165
|
|
| 元素分析 |
C, 72.34; H, 7.99; N, 4.44; O, 5.07; S, 10.16
|
|
| CAS号 |
99755-59-6
|
|
| 相关CAS号 |
Rotigotine Hydrochloride; 125572-93-2; Rotigotine-d7 hydrochloride
|
|
| PubChem CID |
59227
|
|
| 外观&性状 |
White to off-white powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
470.1±45.0 °C at 760 mmHg
|
|
| 闪点 |
238.1±28.7 °C
|
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
|
| 折射率 |
1.611
|
|
| LogP |
4.96
|
|
| tPSA |
51.71
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
3
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
22
|
|
| 分子复杂度/Complexity |
337
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
S1C([H])=C([H])C([H])=C1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])[H])[C@]1([H])C([H])([H])C2C([H])=C([H])C([H])=C(C=2C([H])([H])C1([H])[H])O[H]
|
|
| InChi Key |
KFQYTPMOWPVWEJ-INIZCTEOSA-N
|
|
| InChi Code |
InChI=1S/C19H25NOS/c1-2-11-20(12-10-17-6-4-13-22-17)16-8-9-18-15(14-16)5-3-7-19(18)21/h3-7,13,16,21H,2,8-12,14H2,1H3/t16-/m0/s1
|
|
| 化学名 |
(6S)-6-[propyl(2-thiophen-2-ylethyl)amino]-5,6,7,8-tetrahydronaphthalen-1-ol
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.92 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.92 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.92 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1699 mL | 15.8494 mL | 31.6987 mL | |
| 5 mM | 0.6340 mL | 3.1699 mL | 6.3397 mL | |
| 10 mM | 0.3170 mL | 1.5849 mL | 3.1699 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Rotigotine Versus Placebo, A Study To Evaluate The Efficacy In Advanced Stage Idiopathic Parkinson's Disease Patients
CTID: NCT01646255
Phase: Phase 3 Status: Completed
Date: 2018-04-04
|
|---|
|
 |