| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
PARP-1 ( Ki = 1.4 nM ); PARP-2; PARP-3
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Rucaparib 是纯化全长人 PARP-1 的有效抑制剂,对 LoVo 和 SW620 细胞中的细胞 PARP 显示出更高的抑制作用。此外,Rucaparib 可检测地与其他 8 个 PARP 结构域结合,包括 PARP2、3、4、10、15、16、TNKS1 和 TNKS2。 Rucaparib 的放射增敏作用是由于下游 NF-κB 激活的抑制,并且与 SSB 修复抑制无关。 Rucaparib 可以靶向由 DNA 损伤激活的 NF-κB,并克服经典 NF-κB 抑制剂观察到的毒性,而不损害其他重要的炎症功能。在透化的 D283Med 细胞中,浓度为 1 μM 的 Rucaparib 可抑制 PARP-1 活性 97.1%。激酶测定:测量[32P]NAD+掺入对人全长重组PARP-1的抑制。使用 PhosphorImager 对掺入酸不溶性材料中的 [32P]ADP-核糖进行定量。 Ki是通过非线性回归分析计算的。细胞测定:在过表达人(h) ABCB1的MDCKII亲本细胞系中,rucaparib的顶部和基底外侧定向易位是相同的。用 ABCB1 抑制剂 zosuquidar 处理细胞导致顶端定向转运略有减少,这可能是由于基底外侧未识别的 rucaparib 摄取转运蛋白的特异性抑制,或内源性犬 ABCB1 的抑制。结果表明rucaparib是ABCB1的转运底物。
|
||
| 体内研究 (In Vivo) |
Rucaparib 无毒,但在具有 DNA 修复蛋白能力的 D384Med 异种移植物中显着增强替莫唑胺诱导的 TGD。药代动力学研究还表明,Rucaparib在脑组织中被检测到,这表明Rucaparib具有治疗颅内恶性肿瘤的潜力。 Rucaparib 显着增强拓扑替康和替莫唑胺在 NB-1691、SH-SY-5Y 和 SKNBE (2c) 细胞中的细胞毒性。 Rucaparib 增强替莫唑胺的抗肿瘤活性,并表明 NB1691 和 SHSY5Y 异种移植物中的肿瘤完全且持续消退。
|
||
| 酶活实验 |
测量 [32P]NAD+ 掺入诱导的人全长重组 PARP-1 抑制的量。使用 PhosphorImager,测量添加到酸不溶性材料中的 [32P]ADP-核糖的量。非线性回归分析用于计算Ki。
|
||
| 细胞实验 |
MTT 测定用于测量细胞增殖。在 96 孔板中,细胞以 5×103 细胞/ml 的密度接种在 200 μl/孔的体积中。第二天,将不同浓度的 DMSO、BKM120 或 rucaparib 添加到细胞中。四天后,每个孔接受 20 μl MTT (5 mg/ml)。在 37 °C 下额外孵育 4 小时后,测定 490 nm 处的吸光度。 CalcuSyn 软件用于分析生长抑制实验的数据,以确定药物组合的效果。接下来,计算组合指数(CI)。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rucaparib exhibits a linear pharmacokinetic profile over the dose range from 240 mg to 840 mg twice daily. The mean (coefficient of variation [CV]) steady-state rucaparib Cmax is 1940 ng/mL (54%) and AUC0-12h is 16900 h x ng/mL (54%) at the approved recommended dosage. The mean AUC accumulation ratio is 3.5 to 6.2 fold. The median Tmax at the steady state is 1.9 hours, with a range of 0 to 5.98 hours at the approved recommended dosage. The mean absolute bioavailability is 36%, with a range of 30 to 45%. A high-fat meal increased Cmax and AUC0-24h by 20% and 38%, respectively. The Tmax was delayed by 2.5 hours. Following a single oral dose of radiolabeled rucaparib, unchanged rucaparib accounted for 64% of the radioactivity. Rucaparib accounted for 45% and 95% of radioactivity in urine and feces, respectively. The mean (coefficient of variation) apparent volume of distribution is 2300 L (21%). The mean (coefficient of variation) apparent total clearance at steady state is 44.2 L/h (45%). Metabolism / Metabolites In vitro, rucaparib is primarily metabolized by CYP2D6 and, to a lesser extent, by CYP1A2 and CYP3A4. In addition to CYP-based oxidation, rucaparib also undergoes N-demethylation, N-methylation, and glucuronidation. In one study, seven metabolites of rucaparib were identified in plasma, urine, and feces. Biological Half-Life The mean (coefficient of variation) terminal elimination half-life is 26 (39%) hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of rucaparib, abnormalities in routine liver tests were common; serum ALT elevations arising in 74% with values above 5 times the upper limit of normal (ULN) in 13%. Despite the frequency of serum enzyme elevations during therapy in clinical trials, there were no reports of hepatitis with jaundice or liver failure. Subsequent to its approval and more wide scale use, there have been no published reports of clinically apparent liver injury attributed to rucaparib. Thus, rucaparib is a frequent cause of serum enzyme elevations, but has not been linked to significant hepatotoxicity. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of rucaparib during breastfeeding. The manufacturer recommends that breastfeeding be discontinued during rucaparib therapy and for 2 weeks after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Rucaparib is 70% bound to human plasma proteins _in vitro_. Rucaparib preferentially distributed to red blood cells with a blood-to-plasma concentration ratio of 1.8. |
||
| 参考文献 | |||
| 其他信息 |
Pharmacodynamics
Rucaparib is an anticancer agent that exerts cytotoxic effects against cancer cells. It works by inhibiting poly (ADP-ribose) polymerase (PARP), an enzyme that plays a role in DNA repair. Rucaparib inhibits PARP-1, PARP-2, and PARP-3. It also interacts with PARP-4, PARP-10, PARP-12, PARP-15, and PARP-16, but to a lesser extent. In mice, rucaparib accumulated and was retained in tumours, inhibiting PARP enzymes for seven days. Rucaparib decreases tumour growth in tumour cell lines with deficiencies in BRCA1/2 and other DNA repair genes. In addition to PARP inhibition, rucaparib demonstrated PARP-independent cytotoxic mechanisms in cancer cells. When co-administered with other chemotherapeutic agents, rucaparib contributed to synergistic or additive effects _in vitro_ and _in vivo_. There is evidence that rucaparib can sensitize cancer cells to chemotherapy. Rucaparib can also cause vasodilation, which may increase tumour perfusion and enhance the accumulation of cytotoxic drugs in cancer cells. |
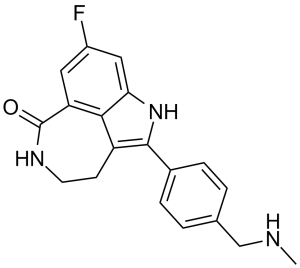
| 分子式 |
C19H18FN3O
|
|---|---|
| 分子量 |
323.37
|
| 精确质量 |
323.143
|
| 元素分析 |
C, 70.57; H, 5.61; F, 5.88; N, 12.99; O, 4.95
|
| CAS号 |
283173-50-2
|
| 相关CAS号 |
1859053-21-6 (camsylate); 459868-92-9 (phosphate); 283173-50-2
|
| PubChem CID |
9931954
|
| 外观&性状 |
Yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
625.2±55.0 °C at 760 mmHg
|
| 闪点 |
331.9±31.5 °C
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
| 折射率 |
1.649
|
| LogP |
2.85
|
| tPSA |
56.92
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
3
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
466
|
| 定义原子立体中心数目 |
0
|
| SMILES |
FC1=C([H])C2C(N([H])C([H])([H])C([H])([H])C3=C(C4C([H])=C([H])C(C([H])([H])N([H])C([H])([H])[H])=C([H])C=4[H])N([H])C(=C1[H])C3=2)=O
|
| InChi Key |
HMABYWSNWIZPAG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H18FN3O/c1-21-10-11-2-4-12(5-3-11)18-14-6-7-22-19(24)15-8-13(20)9-16(23-18)17(14)15/h2-5,8-9,21,23H,6-7,10H2,1H3,(H,22,24)
|
| 化学名 |
6-fluoro-2-[4-(methylaminomethyl)phenyl]-3,10-diazatricyclo[6.4.1.04,13]trideca-1,4,6,8(13)-tetraen-9-one
|
| 别名 |
AG014699; PF-01367338; AG 14447; AG 014699; PF 01367338; AG-014699,PF01367338; AG-14447; AG14447; Trade name: Rubraca
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.73 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 30% propylene glycol, 5% Tween 80, 65% D5W: 30mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0924 mL | 15.4622 mL | 30.9243 mL | |
| 5 mM | 0.6185 mL | 3.0924 mL | 6.1849 mL | |
| 10 mM | 0.3092 mL | 1.5462 mL | 3.0924 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03413995 | Recruiting | Drug: Rucaparib | Prostate Cancer Metastatic | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins |
September 10, 2018 | Phase 2 |
| NCT03462212 | Recruiting | Drug: Rucaparib Drug: Paclitaxel |
Advanced (Stage IIIB-C-IV) Ovarian, Primary Peritoneal and Fallopian Tube Cancer |
Fondazione Policlinico Universitario Agostino Gemelli IRCCS |
March 17, 2021 | Phase 1 Phase 2 |
| NCT04826198 | Recruiting | Drug: Rucaparib Drug: Olaparib |
Ovarian Cancer | Gustave Roussy, Cancer Campus, Grand Paris |
October 5, 2020 | Phase 1 Phase 2 |
AG-014699 inhibits Single strand break (SSB) repair to a similar extent regardless of cellular NF-κB status.Oncogene, 2012, 31(2), 251-264. |
|---|