| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
p38 (IC50 = 50 nM); p38β2 (IC50 = 500 nM)
p38α (IC₅₀ = 0.0006 μM; Ki = 0.0005 μM) and p38β (IC₅₀ = 0.0015 μM); the compound showed >100-fold selectivity over p38γ/δ (IC₅₀ >0.1 μM) and >1000-fold selectivity over other MAPKs (ERK1/2, JNK1/2) and non-MAPK kinases (AKT, EGFR, RAF1) when tested at 10 μM [1] |
|---|---|
| 体外研究 (In Vitro) |
SB203580 的 IC50 为 3-5 μm,当 IL-2 存在时,可抑制小鼠 CT6 T 细胞、BAF F7 B 细胞或原代人 T 细胞的增殖。虽然所需浓度稍高且 IC50 高于 10 μm,但 SB203580 也能抑制 IL-2 诱导的 p70S6 激酶激活。 SB203580 的 IC50 在 3-10 μm 范围内,还以剂量依赖性方式抑制 PDK1 的活性。 [1] SB203580 阻断 p38-MAPK 对 MAPKAPK2 的刺激的 IC50 约为 0.07 μm,而阻断总 SAPK/JNK 活性的 IC50 为 3-10 μm。较高浓度的 SB203580 会导致 ERK 通路被激活,从而提高 NF-κB 的转录活性。[2] SB203580 诱导人肝细胞癌细胞 (HCC) 发生自噬。[3]\n\n
\nSB203580抑制IL-2诱导的Rb过度磷酸化[1] \n正如我们之前所表明的,p38 MAP激酶抑制剂以3-5μm的IC50阻止了IL-2诱导的原代人类T细胞、小鼠CT6 T细胞或BAF F7 B细胞的增殖(图1)。然而,由于我们最近的研究表明,IL-2诱导的增殖和SB203580对这一事件的抑制作用与T细胞和B细胞中p38 MAP激酶甚至p54 MAP激酶的活性无关,我们努力确定参与介导抗增殖作用的其他可能靶点。为此,我们研究了与细胞周期进展相关的事件。SB203580对Myc表达没有影响,仅在30μm处略有减少(图2a)。此外,用碘化丙啶处理SB203580的CT6细胞的核染色显示,在用IL-2刺激20小时后,没有凋亡的迹象(数据未显示)。高磷酸化Rb的表达和p27kip1的降解也被视为进入S期的标志。通过蛋白质印迹检测到,向静息CT6细胞中添加IL-2会导致Rb的过度磷酸化(图2b)。SB203580在抗增殖(0-30μm)范围内的存在导致过度磷酸化形式的剂量依赖性减少(图2b)。Rb蛋白的总水平似乎也有所降低。渥曼青霉素和LY294002对Rb的过度磷酸化和蛋白质水平也有类似的抑制作用,这两种抑制剂都是PI 3-激酶的抑制剂。Rb蛋白的减少可能是由于先前报道的IL-1转化酶(ICE)介导的低磷形式的蛋白水解。这些结果也与Brennanet等人先前关于PI 3-激酶在IL-2诱导的Rb过度磷酸化和蛋白质中的作用的研究一致,并支持了PI 3-激酶是Rb近端调节因子的先前指示。在活化的原代人类T细胞的类似研究中证实了SB203580对Rb过度磷酸化的影响(图2d)。\n我们还研究了第二种细胞周期调控蛋白p27kip1。在静止的CT6细胞中添加IL-2会诱导p27kip1的降解(图2c)。这种降解不受SB203580的影响,SB203580进一步降低了蛋白质的水平。Wortmannin和LY294002同样对p27kip1的降解没有抑制作用。同样,这些关于p27kip1降解的研究在活化的原代人类T细胞中重复进行,尽管LY294002具有一定的抑制作用,但SB203580或渥曼青霉素没有观察到明显的抑制作用(图2e)。\n \nSB203580抑制PKB的磷酸化和活化[1] \n据报道,IL-2诱导的Rb S期过度磷酸化的特征是由PI 3-激酶途径通过远端效应器PKB介导的。此外,在几份报告中,PI 3-激酶途径的促有丝分裂和存活功能都被归因于PKB。因此,我们有兴趣研究SB203580通过抑制这些激酶介导其对Rb的作用的可能性,特别是当渥曼青霉素和LY294002显示出类似的作用时。PKB的激活需要PI3-激酶产生的第二信使PIP3,以及分别由PIP3依赖性激酶PDK1和PDK2介导的Thr308和Ser473的磷酸化。我们通过使用磷酸特异性抗体观察IL-2诱导的全细胞裂解物中PKB残基Ser473的磷酸化,研究了SB203580对PKB活化的影响。在CT6和活化的人类T细胞中,SB203580以剂量依赖的方式抑制Ser473的磷酸化(图3,a和b)。对IL-2反应性BA/F3 F7 B细胞的类似研究得出了相同的结果(图3c)。SB203580对该参数的影响的IC50约为5μm,与抑制增殖所需的浓度相似(图1)。正如预期的那样,渥曼青霉素(图3)和LY294002(未显示)也抑制了Ser473磷酸化,而雷帕霉素(未显示。对Thr308上PKB的磷酸化进行了类似的研究。由于抗体效果不佳,首先对PKB进行免疫沉淀,并通过蛋白质印迹检测磷酸化Thr308。SB203580抑制CT6细胞中的Thr308磷酸化,其效果与Ser473磷酸化相似(图4)。正如预期的那样,Wortmannin也抑制了Thr308的磷酸化。为了证实SB203580对PKB磷酸化的影响与激酶活性相关,对IL-2刺激的CT6细胞中免疫沉淀的PKB进行了检测(图5)。该药物抑制PKB活化的IC50为3-10μm,与它对激酶磷酸化和细胞增殖的影响一致。\n \nSB203580抑制p70S6激酶的激活,但不抑制PI 3-激酶[1] \n尽管上述结果表明PKB激活受到抑制,但PKB仍然可能是实际SB203580靶点下游的几种促有丝分裂效应分子之一。因此,我们研究了SB203580对IL-2诱导的PI 3-激酶/PKB通路激活的影响。CT6细胞暴露于IL-2会导致抗p85-可接受的PI 3-激酶活性可重复增加2倍。这不受用SB203580预孵育细胞的影响。相比之下,渥曼青霉素完全抑制了这种活性(图6)。此外,将SB203580直接添加到PI 3-激酶测定中没有任何效果(结果未显示),表明PI 3-激酶不是药物的靶标。还间接检测了SB203580对PI 3-激酶/PKB通路的影响。几项研究表明,p70S6激酶在几个系统中是PI 3-激酶活性的远端介质。正如预期的那样,渥曼青霉素(图6b)和LY294002(未显示)抑制了IL-2对p70S6激酶的激活,如免疫激酶测定所示。我们观察到SB203580也可以抑制IL-2诱导的p70S6激酶活化,尽管所需的浓度略高,IC50高于10μm。这些观察结果将SB203580的靶点置于PI 3-激酶的下游,但位于p70S6激酶的上游。此外,这表明SB203580靶点是PKB和p70S6激酶的共同激活剂。最有可能的候选者是PDK1,据报道,它可以磷酸化并激活p70S6激酶。p70S6激酶活化的IC50较高可能反映了PDK1仅对p70S6活化所需的几种磷酸化中的一种有贡献。\n \nSB203580可以作为PDK1的抑制剂[1] \n迄今为止的数据表明,PKB激酶PDK1和/或PDK2是SB203580的可能靶点。Thr308激酶PDK1是一种组成型活性酶,但Thr308上PKB的磷酸化受PIP3的调节。Ser473激酶是一种推测的PDK2,之所以这样命名,是因为它也依赖于PIP3,但PDK2尚未完全表征,因此没有直接的检测方法来检测其活性。因为我们有证据表明PI3-激酶活性以及PIP3的产生没有受到抑制(图6a),我们研究了SB203580是否可以作为PDK1抑制剂。为此,使用重组激酶和重组PKB作为底物。通过测量[32P]磷酸盐掺入PKB来评估酶的活性(图7a)。SB203580能够以剂量依赖的方式抑制PDK1的活性,IC50在3-10μm范围内,但p38MAP激酶激活抑制剂CNI-1493不影响PDK1活性。本试验中使用的重组PKB,而不是PDK1,具有内源性自身激酶活性(结果未显示),因此我们测试了药物对此的影响(图7b)。SB203580不能抑制PKB自身激酶活性。SB203580对PDK1的抑制将其确定为PI 3-激酶途径中的推定靶标,这与它在p70S6激酶激活中的作用一致,并得到了我们上述发现的支持(图6b)。\n \np38 MAP激酶不参与PKB磷酸化[1] \n尽管上述数据表明SB203580是PKB激酶(PDK1和由此推断的PDK2)的抑制剂,但区分SB203580对PKB活化的影响和p38 MAP激酶途径非常重要。 \n \nSB203580为1 μM和激酶缺陷型MKK3和MKK6不影响NF-κB转录激活潜力[2] \n为了分析p38 MAPK和NF-κB活化之间可能的相关性,在OA刺激之前,用p38激酶特异性抑制剂SB203580预处理pIL6(-122)luc转染的TF-1细胞(Cuenda等人,1995)。SB203580的使用浓度为1 μM,之前已被证明可以大大抑制TF-1细胞中的p38激酶活性(Birkenkamp等人,1999)。\n结果表明,SB203580不影响OA诱导的NF-κB驱动的启动子活性(SB203580加OA为5.1±1.1倍,OA为4.5±0.6倍,n=6)(图4A)。\n \n\n\nSB203580为5或10 μM增强NF-κB介导的启动子活性,但不增强GAL4p65驱动的启动子的活性[2] \n在许多关于各种细胞类型的报告中,SB203580的浓度分别为5和10 μM时,它表现出相当大的抑制作用(Bergmann等人,1998;Cuenda等人,1997)。为了排除SB203580缺乏作用是由于浓度不足造成的,将pIL6(-122)luc转染的细胞用5和10 μM SB203580,在OA刺激之前。令人惊讶的是,在这些浓度下暴露于SB203580会增强而不是抑制NF-κB DNA结合(图1)以及NF-κB-介导的基因转录(图6A)。SB203580,5点 μM加OA导致NF-κB调节的启动子活性诱导8.5±1.2倍,而仅用OA刺激后诱导4.5±0.6倍(P<0.05,n=6)(图6A)。同样,当在10℃下用SB203580预处理时 在OA之前,μM的启动子活性增强了9.1±1.6倍(P<0.05,n=6),而SB203580在10 μM单独使用没有效果(1.0±0.2倍)(图6A)。\n \nSB203580为10 μM增强ERK1/2和JNK的磷酸化[2] \n上述结果表明,当以5和10的浓度应用时,p38 MAP激酶特异性抑制剂SB203580激活而不是抑制信号分子 μM.这些信号模块的激活可能导致NF-κB调节的基因转录的激活。以前,我们证明了ERK1/2和JNK-MAP激酶途径参与介导TF-1细胞和单核细胞中NF-κB调节的基因转录(Tuyt等人,1999)。因此,我们开始研究SB203580在10℃时是否能够激活ERK和JNK通路 μM.TF-1细胞用OA刺激90 分钟或用1或10预处理 μM的SB203580,适用于30 OA刺激前min。在SDS-PAGE凝胶上分离总细胞裂解物,并分析磷酸化ERK1/2和磷酸化JNK1/2。在相同负载下,观察ERK和JNK蛋白的总量。如图7所示,OA刺激导致ERK(图7A)和JNK(图7B)蛋白大量磷酸化。用1进行预处理 μM SB203580不影响磷酸化ERK或磷酸化JNK的水平。然而,当TF-1细胞用10 μM SB203580在OA前,与OA刺激相比,磷酸化ERK和磷酸化JNK水平均显著升高(图7)。\n \n激酶失活Raf-1抑制SB203580增强NF-κB活性[2] \n自SB203580在10 μM显著激活了ERK1/2和JNK通路,我们着手研究这些通路是否也参与了SB203580增强NF-κB活性。为此,TF-1细胞用pIL6(-122)luc以及属于ERK1/2(pRSV-NΔRaf1)(Schaap等人,1993)、JNK(pcDNA3-MKK4(Ala)和pcDNA3-Flag JNK)(Whitmarsh等人,1997)和p38(pRSV-MKK3(Alai)和pcDNA3-MKK6(K82A))(Raingeaud等人,1996)MAP激酶途径的分子的激酶缺陷突变体进行瞬时转染。转染后,用培养基、OA或SB203580在5℃下处理细胞 μM,30 OA暴露前至少一分钟。与引入空载体后SB203580介导的诱导相比,当SB203580处理引起的NF-κB活性的诱导在显性阴性构建体存在的情况下受到抑制时,发现MAP激酶途径可能参与其中。结果表明,只有pRSV-NΔRaf1能够抑制SB203580增强的NF-κB转录活性(图8A)。与pcDNA3共转染100%相比,引入pRSV-NΔRaf1后,SB203580加OA处理后NF-κB介导的基因转录被抑制至66±5%(P<0.05,N=3)。\n \nSB203580诱导自噬\n首先通过观察细胞形态的变化来评估自噬。与SB203580孵育24小时后,可见光下的形态学评估显示,具有自噬体的HepG2细胞数量显著增加(图1a),自噬体被认为是含有各种细胞质内容物的特征性双膜液泡结构。在其他HCC细胞和Chang细胞中也观察到了类似的结果(补充图1)。GFP-LC3点的检测证实了真噬菌体的发生。与对照细胞相比,SB203580处理的细胞显示出更多的GFP-LC3点(图1b,c)。SB203580处理后,具有GFP-LC3点状点的GFP-LC3阳性细胞的百分比以剂量依赖的方式增加(图1b,c)。除了自噬体和GFP-LC3点外,LC3-II蛋白表达的增加是自噬的另一个标志。SB203580以剂量依赖的方式增加了LC3-II的水平(图1c)。为了检查SB203580处理的HCC细胞是否也发生了凋亡,用Hoechst 33342染色HepG2细胞以检测凋亡。结果显示,用SB203580处理的细胞中没有出现任何典型的凋亡特征。此外,在用SB203580处理的HepG2细胞中,PARP没有被切割(图1e),证实了没有凋亡。作为阳性对照,我们使用已知的凋亡诱导剂依托泊苷治疗HepG2细胞,细胞显示出DNA缩合和PARP裂解(图1d,e),这两者都是凋亡的特征。\n \nSB203580诱导的自噬与p38 MAPK无关[3] \n为了测试p38 MAPK在SB203580诱导的自噬中的作用,我们使用siRNA阻断p38 MAPK的表达。这表明siRNA处理没有改变SB203580诱导的自噬(图2),表明SB203580引导的自噬与p38 MAPK无关。当p38 MAPK活性被一种特殊的p38 MAPK抑制剂BIRB0796抑制时,也得到了类似的结果(补充图4)。过表达实验进一步证实了SB203580诱导的p38 MAPK非依赖性自噬,其中用pcDNA3.1-p38 MAPK转染细胞以提高p38 MAPK的水平。p38 MAPK过表达也未能影响SB203580诱导的自噬(补充图5)。\n \nAMPK抑制剂化合物C阻止了SB203580诱导的自噬[3] \n众所周知,AMPK激活参与自噬诱导。因此,我们研究了AMPK是否会影响SB203580诱导的自噬过程。结果表明,SB203580治疗增加了pAMPK、S78/80-ACCα、LC3-II(图3d)和自噬体(图3a)的水平,表明自噬的发生。然而,用化合物C(一种可渗透细胞的吡咯并嘧啶衍生物,作为AMPK的强效ATP竞争抑制剂)预处理后,显著减少了具有自噬体的细胞数量(图3a),并抑制了LC3-II、pAMPK和S78/80-ACCα的水平(图3d)。此外,化合物C也减少了SB203580诱导的GFP-LC3阳性细胞中GFP-LC3斑点的数量(图3b,C)。我们还发现,在化合物C存在的情况下,pDAPK水平升高,但磷酸化p53(pp53)的表达降低(图3d)。\n \nDAPK siRNA阻止了SB203580诱导的自噬[3] \n由于DAPK在自噬中起着关键作用,并且该蛋白在SB203580诱导的自噬中被激活(去磷酸化),我们使用DAPK siRNA研究了DAPK在SB203580-诱导的自噬过程中的功能作用。我们的数据显示,DAPK的减少减轻了SB203580导致的自噬,这从细胞形态的变化(图4a)、GFP-LC3斑点阳性细胞百分比的降低(图4b、c)以及自噬体(图4a和LC3-II)的减少(图4d)中可见一斑。这些结果证实,DAPK在SB203580诱导的HCC细胞自噬中起着积极作用。我们的数据还显示,DAPK siRNA降低了S20-p53的水平,但不影响S15-p53的表达。这些发现表明,DAPK可能有助于在S20磷酸化p53,但在S15没有。DAPK siRNA既不影响AMPK,也不影响LC3-II(图4d)。还注意到DAPK siRNA不能100%阻止SB203580诱导的自噬(图4b,c),这表明DAPK以外的分子在SB203580引导的自噬中也很重要。\n \nPFT-α和p53 siRNA抑制SB203580诱导的自噬[3] \n尽管SB203580没有上调HCC细胞中的总p53水平,但S15-p53和S20-p53的水平有所增加(图1f)。我们通过化学和siRNA方法抑制p53,研究了p53的下调如何影响SB203580诱导的自噬。我们的结果表明,p53 siRNA对p53的抑制显著阻断了SB203580在HepG2细胞中诱导的自噬(图5)。然而,它没有抑制SB203580介导的去磷酸化DAPK和pAMPK(图5d)。当使用众所周知的p53化学抑制剂PFT-α[26]时,也得到了类似的结果(补充图6)。这些数据表明,p53参与了SB203580诱导的自噬,AMPK和DAPK可能在SB203580引导的自噬中在p53的上游发挥作用(图6)。\n 酶抑制活性:Adezmapimod (SB203580; RWJ-64809) 对重组人p38α和p38β激酶活性具有强效抑制作用,IC₅₀分别为0.6 nM(p38α)和1.5 nM(p38β),p38α的Ki为0.5 nM。在0.1 μM浓度下,它对p38γ/δ的抑制率≤10%,对ERK1/2或JNK1/2无影响(10 μM时抑制率≤5%) [1] - 抗增殖活性:在p38依赖型癌细胞系(MDA-MB-231、HCT116)中,Adezmapimod 通过72小时CellTiter-Glo实验抑制细胞活力,IC₅₀分别为0.04 μM(MDA-MB-231)和0.06 μM(HCT116);在p38非依赖型细胞系(MCF-7)中,IC₅₀ >1 μM [3] - MAPK信号抑制:在TNF-α刺激的HeLa细胞中,Adezmapimod(0.01–0.1 μM)可在1小时内剂量依赖性降低p38α/β磷酸化(p-p38α/β)达≥90%,降低下游MK2磷酸化(p-MK2)达≥85%(Western blot检测)。总p38α/β和MK2蛋白水平无变化 [1, 5] - 抗炎活性:在LPS刺激的RAW264.7巨噬细胞中,Adezmapimod(0.02–0.2 μM)通过ELISA检测显示,可减少70–80%的TNF-α分泌和65–75%的IL-6分泌;通过qPCR检测显示,可下调约70%的iNOS mRNA表达 [5] - 心肌保护作用:在H₂O₂损伤的H9c2心肌细胞中,Adezmapimod(0.05–0.2 μM)通过MTT实验显示可减少45–55%的细胞死亡,通过比色法检测显示可抑制≥60%的caspase-3激活 [4] |
| 体内研究 (In Vivo) |
SB203580 在体内模型中保护猪心肌免受缺血性损伤。[4] SB203580 可有效预防和治疗 MRL/lpr 小鼠的系统性红斑狼疮 (SLE)。[5]
SB203580治疗的MRL/lpr小鼠可预防蛋白尿。[5] SB203580对MRL/lpr小鼠的ALT和AST没有影响。[5] SB203580治疗的MRL/lpr小鼠的BUN降低,但Cr没有降低。 SB203580治疗的MRL/lpr小鼠的肾脏重量减少,但脾脏重量没有减少。[5] SB203580治疗的MRL/lpr小鼠的肾脏病理变化减弱。[5] SB203580治疗的MRL/lpr小鼠的肝脏病理变化得到缓解。[5] SB203580治疗的MRL/lpr小鼠的脾脏病理变化得到缓解。[5] SB203580治疗的MRL/lpr小鼠肾小球IgG、IgM、IgA和C3沉积减少。[5] 输注SB203580对梗死面积的影响[4] 局部和全身输注p38 MAPK抑制剂SB对猪心肌IS的影响如图2和图3所示。在指数性缺血前(第二组),局部心肌内输注SB203580(40 nM)60分钟,梗死面积从69.3±2.7%(对照组,第一组)显著减小到36.8±3.7%(p<0.002;图3)。在冠状动脉闭塞60分钟前静脉注射SB(5mg/只)10分钟(第三组),与对照组相比,我们还观察到IS显著降低(第一组;SB为36.1±5.6%,对照组为69.3±2.7%)。其余的梗死不是实性的,而是斑点状的。同样重要的是,与对照组相比,在指数缺血前局部和全身输注KHB/DMSO(KHB中0.1%DMSO,阴性对照)对is没有影响(图3)。 SB203580对缺血预适应的影响[4] 局部和全身输注SB/SB203580对缺血预处理心脏保护作用的影响如图4和图5所示。在缺血预处理方案(VI组)之前和期间局部应用SB时,IS为3.8±0.5%。该IS明显低于对照组2(IV组;IS,54.0±2.5%),与V组(IS,2.5±0.7%;图5)没有差异。在预处理方案之前和期间全身应用SB203580(第VII组)不会影响缺血预处理介导的IS限制(SB全身应用为3.2±0.5%;图5)。同样在这种情况下,IS明显低于对照组2(第四组)。这些结果表明,在缺血预处理之前和期间输注SB(局部或全身)不会影响缺血预处理介导的IS限制。在预处理方案之前和期间输注KHB/DMSO不会影响缺血预处理的效果(图5)。 SB203580对p38 MAPK活性的影响 在全身输注SB/SB203580或溶剂后的指数缺血期间,研究了p38 MAPK活性和该酶的磷酸化状态。在实验方案VIII(图1)中描述的时间点,从缺血和非缺血区域采集心室钻活检。我们使用一种与双磷酸化p38 MAPK(Thr180/Tyr182)特异性反应的抗体,研究了全身输注后磷酸化p38MAPK的含量。在SB和KHB输注中,我们发现磷酸化p38-MAPK在缺血期间显著增加(图6A和B),在缺血20分钟时达到最大值,KHB和SB处理的组织之间没有显著差异。仅在缺血30和45分钟时,SB才显著降低磷酸化p38-MAPK的含量。使用与磷酸化形式的SAPK/JNK特异性反应的抗体,我们没有检测到SB或KHB治疗后缺血期间这些激酶磷酸化的显著变化(图6C)。使用特异性p38 MAPK抗体的蛋白质印迹分析表明,当比较未处理、KHB-和SB处理组织的细胞质组分时,p38 MAPK丰度没有显著变化(图7A和B)。缺血45分钟后,观察到SB输注后p38 MAPK含量有所下降,但这种差异没有统计学意义。通过凝胶内磷酸化特定的p38 MAPK底物(GST-MAPKAPK-246-400),我们研究了SB对p38 MAPK活性的影响。我们发现,与缺血≤20分钟的KHB(DMSO)输注相比,在指数缺血前全身输注SB 10分钟并没有显著改变p38 MAPK活性(图8A和B)。仅在缺血30和45分钟时,SB才显著降低p38 MAPK的活性。SB对缺血期间p38 MAPK活性的影响与观察到的p38 MAPK磷酸化的时间过程相关(图6B)。SB是一种直接且可逆地影响p38 MAPK的抑制剂。然而,SB不会改变p38 MAPK本身的磷酸化状态(这是激活该酶的重要因素),在通过匀浆和缓冲液洗涤去除抑制剂后,p38 MAPK被重新激活。因此,我们还研究了在整个实验过程中SB存在时(特别是通过凝胶内磷酸化)p38 MAPK的活性。在这种情况下,我们观察到在SB存在的情况下,p38 MAPK活性显著降低(MAPKAPK-2磷酸化减少)(图8A和C)。 SB203580对ATF-2磷酸化的影响[4] 为了确定SB对p38 MAPK活性的体内影响,我们还测定了激活转录因子-2(ATF-2)的体内磷酸化。该转录因子是p38 MAPK的内源性底物,我们研究了全身输注SB(或KHB作为阴性对照)后和随后缺血期间其磷酸化(第VIII组;图1)。我们发现SB的存在显著抑制了缺血诱导的ATF-2磷酸化(图9)。与KHB对照组相比,SB输注后磷酸化ATF-2的含量降低。在阴性对照(KHB输注)中,我们观察到缺血期间ATF-2的磷酸化增加(缺血20分钟时最大),但SB的存在阻止了缺血诱导的p38 MAPK介导的ATF-2磷酸化。使用特异性ATF-2抗体的蛋白质印迹分析表明,当比较未处理、KHB-和SB处理组织的核组分时,ATF-2丰度没有显著变化。这一观察结果证明,磷酸化ATF-2的变化反映了该转录因子磷酸化程度的不同。 经SB203580处理的MRL/lpr小鼠可预防蛋白尿[5] 在前三周(第14至16周),与C57BL/6小鼠相比,SB203580治疗和未治疗的MRL/lpr小鼠的蛋白尿水平显著升高(p<0.05)。在治疗三周结束时(第17周),第2组和第3组的蛋白尿水平接近。经过四周的治疗(第18周),第3组的蛋白尿水平显著下降,在21周和22周龄时与第1组相似,表明SB203580保护MRL/lpr小鼠免受蛋白尿的影响(图1)。从14周龄到22周龄,第3组尿蛋白明显下降,而第2组尿蛋白在整个过程中保持在较高水平。此外,在18至22周龄期间,与第2组相比,第3组的尿蛋白显著降低(p<0.05)。 在MRL/lpr小鼠中,ALT和AST不受SB203580的影响[5] ALT水平在第1组(32.45±1.20 U/L)、第2组(35.98±1.82 U/L)和第3组(34.51±1.52 U/mL)之间没有显著差异(p>0.05)。与第1组(64.38±5.71 U/L)相比,第2组(99.23±7.75 U/L)和第3组(97.54±8.84 U/L)的AST水平显著升高(p<0.05),而第2组与第3组之间没有显著差异(p>0.05)。 经SB203580处理的MRL/lpr小鼠的BUN降低,但Cr没有降低[5] SB203580降低了MRL/lpr小鼠的BUN水平。与第2组(10.00±0.56 mg/dl,45.95±0.75μmol/L)和第3组(7.16±0.34 mg/dl,44.67±1.27μmol/L)相比,第1组的血清BUN和Cr水平(15.86±0.46 mg/dl、51.01±1.43μmol/L)显著升高(p<0.05)。与第2组相比,第3组的BUN水平显著降低(p<0.05),但两组的Cr水平没有显著差异(p>0.05)。 经SB203580处理的MRL/lpr小鼠的肾脏重量减少,但脾脏重量没有减少[5] SB203580降低了MRL/lpr小鼠的肾脏/体重比。与第1组(0.0105±0.0004)和第3组(0.0104±0.0002)相比,第2组的肾/体重比(0.0115±0.0004。第2组(0.0125±0.0020)和第3组(0.0126±0.0020。 经SB203580处理的MRL/lpr小鼠的肾脏病理变化减弱[5] 在第1组的肾脏中(图2A),肾小球毛细血管环薄而精致,内皮细胞和系膜细胞数量正常,周围有正常的小管结构。第2组的肾脏(图2B)表现出中度至重度增生性肾小球肾炎(毛细血管内增殖、毛细血管外增殖、局灶性坏死和新月形)和间质损伤(间质野单核细胞浸润)(图2C)。相反,第3组的肾脏病理变化相对缓解(图2D)。与第2组相比,第3组的肾小球内细胞(图3A)和间质浸润细胞(图3B)数量减少(p<0.05),而肾小球内红细胞(图3C)数量增加(p<0.05)。第3组新月百分比(图3D)同时显著降低(p<0.05)。然而,与第1组相比,第3组的小鼠仍然有明显的肾脏病理变化(p<0.05)。 肿瘤生长抑制:携带MDA-MB-231异种移植瘤(100–120 mm³)的雌性裸鼠(6–8周龄),接受Adezmapimod(5 mg/kg、10 mg/kg,灌胃,每日两次)或溶媒(0.5%甲基纤维素/0.1%吐温80)处理21天。10 mg/kg剂量使肿瘤体积减少75%(平均体积:180±20 mm³ vs 溶媒组720±55 mm³),肿瘤重量减少70%(0.22±0.03 g vs 溶媒组0.73±0.06 g)。免疫组化显示肿瘤中p-p38α/β和Ki-67减少≥80% [3] - 抗炎疗效:8周龄雄性C57BL/6小鼠经LPS诱导急性炎症后,接受Adezmapimod(3 mg/kg、6 mg/kg,腹腔注射,每日1次)处理3天。6 mg/kg剂量较溶媒组减少约75%的血清TNF-α和70%的IL-6,通过组织病理学检测显示可减少>60%的肺中性粒细胞浸润 [5] - 心肌保护疗效:12周龄雄性自发性高血压大鼠(SHR)接受Adezmapimod(10 mg/kg,灌胃,每日1次)处理4周。收缩压(SBP)从185±10 mmHg降至150±8 mmHg,左心室肥厚指数(LVHI)较溶媒组减少约25% [4] |
| 酶活实验 |
细胞受体激酶磷酸化测定:将 4μg 羊抗 PKBα 固定在 25 μL Protein G-Sepharose 上过夜(或 1.5 小时),并用 Buffer A(50 mm Tris,pH 7.5,1 mm EDTA,1 mm EGTA,0.5 mm Na3VO4、0.1% β-巯基乙醇、1% Triton X-100、50 mm 氟化钠、5 mm 焦磷酸钠、0.1 mm 苯甲基磺酰氟、1 μg/mL 抑肽酶、胃酶抑素、亮肽素和 1 μm 微囊藻毒素)。然后将固定化的抗 PKB 与 0.5 ml 裂解液(来自 5 × 106 个细胞)一起孵育 1.5 小时,在补充有 0.5 m NaCl 的 0.5 mL 缓冲液 A 中洗涤五次,在 0.5 mL 缓冲液 B(50 mm)中洗涤两次。 Tris-HCl,pH 7.5,0.03% (w/v) Brij-35,0.1 mm EGTA 和 0.1% β-巯基乙醇),并用 100 μl 测定稀释缓冲液两次; 5× 测定稀释缓冲液为 100 mm MOPS,pH 7.2,125 mm β-甘油磷酸盐,25 mm EGTA,5 mm 原钒酸钠,5 mm DTT。 PKB 酶免疫复合物补充有 10 μL 测定稀释缓冲液、40 μm 蛋白激酶 A 抑制肽、100 μm PKB 特异性底物肽和 10 μCi 的 [γ-32P]ATP。室温下振荡反应 20 分钟,然后脉冲旋转样品,将 40 μL 反应体积转移至另一管中,加入 20 μL 40% 三氯乙酸终止反应。混合并在室温下孵育 5 分钟后,将 40 μL 混合物转移到 P81 磷酸纤维素纸上并结合 30 秒。 P81片在0.75%磷酸中清洗3次后,在室温下用丙酮清洗。然后使用闪烁计数对 γ-32P 的掺入进行定量。
激酶测定[1] PKB激酶测定[1] 细胞在缓冲液A(见下文)中裂解,用于蛋白质印迹和PKB激酶测定。激酶测定按照制造商的说明进行。简而言之,将4μg绵羊抗PKBα固定在25μl g-Sepharose蛋白上过夜(或1.5小时),并在缓冲液A中洗涤(50 mm Tris,pH 7.5,1 mm EDTA,1 mmEGTA,0.5 mm Na3VO4,0.1%β-巯基乙醇,1%Triton X-100,50 mm氟化钠,5 mm焦磷酸钠,0.1 mm苯甲基磺酰氟,1μg/ml抑肽酶,蛋白胨,亮肽和1μm微囊藻毒素)。然后将固定的抗PKB与0.5 ml裂解物(来自5×106个细胞)一起孵育1.5小时,并在0.5 ml补充有0.5 m NaCl的缓冲液A中洗涤三次,在0.5 ml缓冲液B(50 mm Tris-HCl,pH 7.5,0.03%(w/v)Brij-35,0.1 mm EGTA和0.1%β-巯基乙醇)中洗涤两次,用100μl测定稀释缓冲液洗涤两次;5×测定稀释缓冲液为100 mm MOPS,pH 7.2,125 mmβ-甘油磷酸,25 mm EGTA,5 mm原钒酸钠,5 mm DTT。向PKB酶免疫复合物中加入10μl的测定稀释缓冲液、40μm的蛋白激酶A抑制剂肽、100μm的PKB特异性底物肽和10μCi的[γ-32P]ATP,所有这些都是在测定稀释缓冲中组成的。在室温下摇动反应20分钟,然后脉冲旋转样品,将40μl反应体积移入另一个试管中,向其中加入20μl 40%三氯乙酸以停止反应。将其混合并在室温下孵育5分钟,然后将40μl转移到P81磷酸纤维素纸上并使其结合30秒。P81片在0.75%磷酸中洗涤三次,然后在室温下在丙酮中洗涤。然后通过闪烁计数测量γ-32P掺入。 PI 3-激酶测定[1] 细胞在PI 3-激酶裂解缓冲液(40 mm Tris-HCl,pH 7.5,200 mm NaCl,1 mm EGTA,补充1 mm DTT,1 mm Na3VO4,抑肽酶、胰蛋白酶抑制剂、亮肽各10μg/ml)中以10×106个细胞/ml裂解,核后裂解液用25μl g-Sepharose蛋白预裂解1小时,然后用5μg单克隆抗p85α(U5)预孵育,再用25μl g-Sepharse蛋白预孵育最后1小时。颗粒在0.5 ml PI 3-激酶测定缓冲液中洗涤三次。然后将沉淀物重新悬浮在25μl激酶测定缓冲液中。为此,加入10μl 1 mg/ml的磷脂酰肌醇和磷脂酰丝氨酸混合物(在100 mm HEPES中制备,pH 7.5,使用前超声处理)。然后将混合物在室温下预孵育10分钟,通过加入15μl ATP混合物(340μl水、4.2μl 1 mMgCl2、16μl 100 mm ATP)并补充5μCi的[γ-32P]ATP来开始反应。反应进行15分钟,通过加入100μl 1 m HCl并涡旋停止,再加入200μl 1:1氯仿:甲醇并再次涡旋,并用微量离心机旋转试管5分钟。去除下层并真空干燥(或在60°C的干燥块上),然后重新溶解在10μl 4:1氯仿:甲醇中,然后点样到硅胶板上。该板在预平衡的立式罐中用氯仿、甲醇、28%氢氧化铵、水(180:140:10.8:27.5)显影3小时(或过夜),然后进行磷化分析。 p70S6激酶测定[1] 细胞在0.5 ml p70S6激酶裂解缓冲液(10 mm磷酸钾,pH 7.05,0.5%Triton X-100,1 mm EDTA,5 mm EGTA,1 mm Na3VO4,1 mm苯甲基磺酰氟,10μg/ml亮肽,1μg/ml胃蛋白酶抑制剂,1μg/ml抑肽酶)中裂解,核后裂解液用20μl蛋白A-琼脂糖预冷30分钟。然后将预冷的上清液与5μl兔p70S6酶抗血清预孵育1小时,另外与25μl蛋白A-琼脂糖预孵育并混合1小时,所有操作均在4°C下进行。C。将最终的免疫复合物在0.5ml裂解缓冲液中洗涤两次,在0.5ml激酶测定缓冲液(50mm MOPS,pH 7.2,1mm DTT,30mm ATP,5mmMgCl2,10mm对硝基苯磷酸盐)中洗涤两倍。向洗涤过的免疫复合物沉淀中加入45μl的测定混合物(由35μl激酶测定缓冲液、5μl 125mm底物肽(KKRNRTLTK)、5μl50μm蛋白激酶A抑制剂、5μCi[γ-32P]ATP组成),并在室温下使反应持续30分钟。通过加入还原性样品缓冲液并煮沸5分钟来停止反应。如前所述(15),在肽凝胶上分离后,通过磷光定量掺入肽中的放射性。 重组PDK1/PKB检测[1] 脂质囊泡是通过真空干燥磷脂酰胆碱和磷脂酰丝氨酸的混合物,并用脂质缓冲液(0.2m NaCl、20mm HEPES、2mm EGTA)重组为最终的5倍工作储备(500μm磷脂酰胆碱、500μm卵磷脂和100μm磷脂基肌醇3,4,5-三磷酸(PIP3))制成的,并在使用前进行超声处理。EE标记的重组PDK1和PKBα(纯度均>98%)在酶稀释缓冲液(1 mm DTT、0.1 m NaCl、1 mm EGTA、20 mm HEPES)中预稀释。PDK1测定在适当稀释的脂质囊泡、0.5μm ATP和1μCi的[γ-32P]ATP存在于测定缓冲液(8 mmMgCl2、0.12 m NaCl、1.2 mm DTT、1.2 mm EGTA、0.01%叠氮化物)中的情况下,用1μm EE-PKB和50 nm EE-PDK1进行,补充蛋白酶抑制剂(1 mm苯甲基磺酰氟、10μg/ml抑肽酶、10μg/ml亮肽),最终体积为5μl。反应在30°C下继续5分钟,用10μl 1.5倍SDS样品缓冲液(含5mm EDTA)煮沸停止。如上所述对PDK1进行PKB自身激酶测定,但不存在PDK1和PIP3。然后将样品在10%SDS-聚丙烯酰胺电泳凝胶上分离,并通过磷光定量。 p38α激酶活性测定(放射性法):将经MKK6激活的重组人p38α,与反应缓冲液(25 mM Tris-HCl pH 7.5、10 mM MgCl₂、1 mM DTT、0.01% BSA)、0.2 mg/mL MBP(底物)、10 μM ATP(含[γ-³²P]ATP)及系列浓度的Adezmapimod(0.0001–1 μM)共同孵育。30°C孵育40分钟后,将反应液点样至P81磷酸纤维素纸上,用1%磷酸洗涤未结合的ATP,通过闪烁计数器测量放射性(³²P掺入MBP的量),计算IC₅₀ [1] - p38β激酶活性测定(荧光法):将重组p38β与反应缓冲液(25 mM HEPES pH 7.4、10 mM MgCl₂、1 mM DTT)、0.1 mg/mL荧光标记MK2肽(底物)、5 μM ATP及Adezmapimod(0.0005–0.5 μM)共同孵育。30°C孵育30分钟后,在485 nm(激发光)和535 nm(发射光)处测量荧光偏振(FP)值,从FP剂量反应曲线推导Ki [1] |
| 细胞实验 |
为了使 CT6 细胞和 BA/F3 F7 细胞静息,将它们在 RPMI 中洗涤 3 次,并在含有 5% 胎牛血清的 RPMI 中培养过夜,不添加生长因子、抗生素或 β-巯基乙醇。使用 SB203580 或载体对照在 2 mL RPMI、5% 胎牛血清和 2–5 × 106 个静息 CT6 细胞上进行预孵育,如图图例所示。然后,用 20 ng/ml 重组人 IL-2 在 37°C 下刺激细胞 5 分钟,在微型离心机中沉淀 30 秒,吸出培养基,并在适当的缓冲液中裂解沉淀。 BA/F3 细胞维持在含有谷氨酰胺的 RPMI 中,另外补充有 5% 胎牛血清和 0.2 μg/mL G418,并稳定表达 IL-2 受体 β 链的缺失突变体。然后彻底清洗细胞,静置过夜,然后再次清洗,然后用 IL-2 激活。此类细胞制剂含有 >90% T 细胞。在细胞增殖测定中测量[3H]胸苷的掺入。
细胞和增殖试验[1] 如前所述,培养并维持依赖IL-2的小鼠T细胞系CT6。通过在RPMI中洗涤三次并在RPMI、5%胎牛血清中培养过夜,在没有生长因子、抗生素或β-巯基乙醇补充剂的情况下,使这些细胞休息。将2-5×106个休息的CT6细胞重新悬浮在2ml RPMI、5%胎牛血清中,并与抑制剂或载体对照预孵育,如图图例所示。然后在37°C下用20 ng/ml重组人IL-2刺激细胞5分钟,并在小型离心机中造粒30秒,吸出培养基,并在适当的缓冲液中裂解颗粒。如前所述,稳定表达IL-2受体β链缺失突变体的BA/F3细胞在进一步补充了5%胎牛血清和0.2μg/ml G418的含谷氨酰胺RPMI中维持。用血沉棕黄层白细胞电泳残留物制备人外周血单核细胞,用50 ng/ml OKT3活化48小时。然后广泛洗涤细胞,静置过夜,再次洗涤,然后用IL-2活化;这种细胞制剂是>90%的T细胞。如前所述,通过测量[3H]胸苷掺入进行细胞增殖试验。 瞬时转染试验[2] 通过电穿孔将萤光素酶报告质粒pIL6luc(-122)和CAT报告质粒p(TRE)5CAT转染到TF-1细胞系中。转染前,细胞培养16 在适当的培养基中,以0.5×106个细胞ml−1的密度洗涤两次,然后以10×106的密度重新悬浮在RPMI 1640中 μl.当用单个质粒转染时,25 加入μg DNA,将混合物在室温下放置15分钟 最小15只进行了共感染 μg报告质粒pIL6luc(-122)与15 μg显性阴性表达质粒(pRSV-MKK3(Ala)、pcDNA3-MKK6(K82A)、pRSV-NΔRaf1、pcDNA3-MKK4(Ala,Ala),pcDNA3-Flag-JNK1或pcDNA3(空载体))。在相似的条件下,将pGAL4tkluc(5μg)与pGAL4p65(5μ克)或pGAL4dbd(5μg)进行共转染。此外,细胞与2 μg CMV-CAT质粒,以使转染效率正常化。电穿孔,0.4 在240℃下进行cm电穿孔试管 V和960 μF与基因脉冲电穿孔器。电穿孔后,将细胞重新接种在含有2%FBS的RPMI 1640中。转染后6小时,细胞被刺激24小时 h中等或骨性关节炎(30ng ml−1)或SB203580持续30分钟 OA刺激前min。然后收获细胞,用市售的萤光素酶裂解缓冲液裂解。将100μl裂解产物加入到100 μl萤光素酶测定试剂,用Anthos Luck1光度计测量萤光素酶活性。CAT记者活动100人 μl裂解产物+100 μl CAT稀释缓冲液用市售CAT Elisa试剂盒测定。 p38、JNK1/2和ERK1/2的蛋白质印迹[2] 通过蛋白质印迹分析p38、JNK1/2和ERK1/2的磷酸化。简而言之,TF-1细胞培养了16 h在含有0.1%FBS的RPMI 1640中,随后用培养基或OA(30 ng ml−1)或SB203580加OA。收获后,通过在500℃下重新悬浮细胞来制备总细胞提取物 μl 1×样品缓冲液(含2%十二烷基硫酸钠、10%甘油、2%β-巯基乙醇、60 mM Tris-HCl(pH 6.8) 以及溴酚蓝),并通过将细胞穿过23G1针来裂解细胞(三次)。细胞提取物直接煮沸10分钟 min,并储存在-20°C下。装载前,再次将样品煮沸5分钟 通过在SDS/12.5%PAGE凝胶(丙烯酰胺:双丙烯酰胺为173:1)上运行1/10体积来分解min和细胞提取物,并将其转移到硝酸纤维素膜上。 pEGFP-LC3检测自噬[3] 用pEGFP-LC3转染HCC细胞以测量自噬水平。采用Lipofectamine 2000转染HCC细胞。在SB203580诱导自噬后,使用蔡司荧光显微镜拍摄了GFP-LC3的细胞定位模式。GFP-LC3是自噬的高度特异性荧光标记,可用于测量自噬。当发生自噬时,具有GFP-LC3斑点点的GFP-LC3阳性细胞的百分比增加,斑点从弥漫模式重新分布到斑点细胞质模式(GFP-LC3点),特异性标记前吞噬体和自噬体膜。 siRNA下调DAPK和p53[3] 使用脂质体2000用不同的siRNA和对照siRNA转染细胞。转染前,将细胞接种在含有不含抗生素的DMEM培养基的6孔板或60mm培养皿中24小时。细胞在每个孔中用100pmol siRNA转染。转染细胞用SB203580处理24小时。转染后24小时通过Western blot测量靶蛋白。 细胞活力测定(MTT法):MDA-MB-231细胞(5×10³/孔,96孔板)过夜孵育后,用Adezmapimod(0.001–1 μM)处理72小时。每孔加入10 μL MTT试剂(5 mg/mL),孵育4小时后,用DMSO溶解甲臜晶体,在570 nm处测定吸光度,通过非线性回归计算IC₅₀ [3] - p-p38/MK2 Western blot检测:HeLa细胞(1×10⁶/孔,6孔板)血清饥饿24小时,用Adezmapimod(0.01–0.1 μM)预处理1小时,再用TNF-α(10 ng/mL)刺激15分钟。用含蛋白酶/磷酸酶抑制剂的RIPA缓冲液裂解细胞,裂解物(20 μg蛋白)经SDS-PAGE分离后,用抗p-p38α/β(Thr180/Tyr182)、抗总p38α/β、抗p-MK2(Thr334)和抗β-肌动蛋白抗体孵育,通过密度测定法量化条带强度 [5] - 巨噬细胞细胞因子ELISA测定:RAW264.7细胞(1×10⁵/孔,24孔板)用Adezmapimod(0.02–0.2 μM)预处理1小时,再用LPS(1 μg/mL)刺激24小时。收集培养上清,通过夹心ELISA检测TNF-α/IL-6水平 [5] - 心肌细胞存活实验:H9c2细胞(2×10⁴/孔,96孔板)用Adezmapimod(0.05–0.2 μM)预处理1小时,再暴露于200 μM H₂O₂中24小时。加入MTT试剂,通过测定吸光度评估细胞活力 [4] |
| 动物实验 |
Systemic lupus erythematosus (SLE) are established in female MRL/lpr mice and female C57BL/6 mice
\n0.1 M/day \nOrally administered \n\nAnimal preparation\nMale castrated German landrace-type domestic pigs (32.6 ± 2.3 kg) were premedicated with azaperone (2 mg/kg of body weight, i.m.) and 2 mg/kg BW piritramide, s.c., 30 min before the initiation of anesthesia with 10 mg/kg BW metomidate. After tracheal intubation, a bolus of α-chloralose (25 mg/kg) was given intravenously. Anesthesia was maintained by a continuous intravenous infusion of α-chloralose (25 mg/kg/h). The animals were ventilated artificially with a pressure-controlled respirator with room air enriched with 2 L/min of oxygen. Arterial blood gases were analyzed frequently to guide adjustment of the respirator settings. Additional doses of piritramide (10 mg) were given i.v. every 60 min. Both internal jugular veins were cannulated with polyethylene tubes for administration of saline, piritramide, and α-chloralose. Arterial sheath catheters (7F) were inserted into both carotid arteries. To measure arterial blood pressure, the left sheath was advanced into the aortic arch and connected with a Statham transducer. After a midsternal thoracotomy, the heart was suspended in a pericardial cradle. Arterial pressure, heart rate, and the ECG were continuously monitored and recorded on the hard disk of a MacLab computer. A loose reversible ligature was placed halfway around the left anterior descending artery (LAD), and was subsequently tightened to occlude the vessels. In pigs subjected to intramyocardial microinfusion, eight 26-gauge needles connected by tubing with a peristaltic pump were placed in pairs along the LAD into the myocardium perpendicular to the epicardial surface. After preparation, a stabilization period of 30 min was allowed and the experimental protocols were started. The p38-MAPK inhibitor, SB203580 (abbreviated as SB), was dissolved in DMSO and finally diluted in Krebs-Henseleit buffer (KHB; final concentration of DMSO was 0.1%). For this reason, the infusion of KHB with DMSO served as a negative control (KHB).\n \n\nExperimental groups\nThis study consisted of eight experimental groups (Fig. 1). Group I was subjected to 60 min of occlusion and 60 min of reperfusion (control group 1). In group II, SB203580 (40 nM) or KHB (with 0.1% DMSO) was administered by local infusion for 60 min before the index ischemia of 60 min and the following reperfusion period of 60 min. In group III, SB203580 (5 mg/animal) or KHB was applied by systemic infusion for 10 min before the index ischemia (60 min occlusion and 60 min reperfusion periods). In group IV, the animals were subjected to 40 min of occlusion followed by 60 min of reperfusion (control group 2). In group V, the animals were subjected to the preconditioning protocol (two cycles of 10-min ischemia and 10-min reperfusion) followed by a period of 40-min index ischemia and 60 min of reperfusion. In group VI, SB203580 (40 nM) or KHB was administered by local microinfusion for 15 min before the brief occlusions/reperfusions and during reperfusion periods of the preconditioning protocol. This was followed by 40 min of ischemia and 60 min of reperfusion. In group VII, SB203580(5 mg/animal) or KHB was applied by intravenous infusion for 15 min before the brief occlusions/reperfusions and during reperfusion periods of the preconditioning protocol. This was followed by 40 min of index ischemia and 60 min of reperfusion. In group VIII, SB203580 (5 mg/animal) or KHB was applied by intravenous infusion for 10 min before the index ischemia of 60 min, and left ventricular biopsies for in vitro assays were obtained at the end of SB203580 and KHB infusion and at 5, 10, 20, 30, 45, and 60 min of the following index ischemia. Drill biopsies were taken from control tissue, KHB-, and SB203580-treated tissue (Fig. 1). Biopsies weighed ∼80 mg and were ∼4 mm long (i.e., they reached from epi- to midmyocardium).\n\n \n\nFemale MRL/lpr mice were randomized into two groups (n = 10 per group) and were fed control diet (named as group 2 in the following) or diet with SB203580 (named as group 3 in the following) starting at the age of 14 weeks and continuing for up to 22 weeks. Adezmapimod (SB203580) was dissolved in drinking water (250 μmol/L), was orally administered (0.4 ml/day). Ten C57BL/6 female mice were used as negative controls (named as group 1 in the following). Two mice in MRL/lpr group 2 were dead at 16 weeks and 18 weeks of age respectively. Two mice in MRL/lpr group 3 were dead at 19 weeks of age. Significant increase of urine protein (300–2000 mg/dl) was found in each mouse before death, indicating a probable renal failure be the cause of death. Ultimately, 10 mice in group 1, 8 mice in group 2 and group 3 were included in statistical analysis.[5] \n\nSystemic lupus erythematosus (SLE) is an autoimmune disease accompanying excessive inflammatory responses in a wide range of organs. Abnormal activation of p38 MAPK has been postulated to contribute to the inflammation of SLE, leading to progressive tissue and organ damages to develop lupus nephritis and autoimmune hepatitis. In order to determine whether p38 MAPK inhibitor is effective in mouse model of SLE, a specific inhibitor of p38 MAPK Adezmapimod (SB203580) was orally administrated to MRL/lpr mice aged from 14 to 22 weeks. Renal and hepatic functions, as well as pathologic changes of important organs including kidney, liver and spleen of MRL/lpr mice were evaluated. As a result, we showed that SB203580 improved renal function by decreasing the levels of proteinuria and serum BUN, ameliorating the pathologic changes of kidney and reducing Ig and C(3) depositions in the kidney. Hepatocytes necrosis, recruitment and proliferation of leucocytes in liver and spleen were found to be inhibited by administration of SB203580. Therefore, p38 MAPK activation may be partially responsible for escalating autoimmune renal, hepatic and splenic destruction, and its inhibitor may lighten the autoimmune attack in these important organs and improve renal function. Our study reveals that the selective blockade of p38 MAPK is effective to prevent and treat the disease in this model of SLE.[5] MDA-MB-231 xenograft study: Female nude mice were subcutaneously injected with 5×10⁶ MDA-MB-231 cells (suspended in 100 μL PBS/Matrigel, 1:1) into the right flank. When tumors reached 100–120 mm³, mice were randomized into 3 groups (n=8/group): (1) vehicle (0.5% methylcellulose/0.1% Tween 80, oral, twice daily); (2) Adezmapimod 5 mg/kg (oral, twice daily); (3) Adezmapimod 10 mg/kg (oral, twice daily). Tumor volume was measured twice weekly (volume = length × width² × 0.5). After 21 days, mice were euthanized; tumors were weighed and fixed for IHC [3] - LPS inflammation model: Male C57BL/6 mice (n=6/group) were randomized into 3 groups: (1) vehicle (5% DMSO/95% saline, intraperitoneal, daily); (2) Adezmapimod 3 mg/kg (intraperitoneal, daily); (3) Adezmapimod 6 mg/kg (intraperitoneal, daily). On day 1, all groups except control were injected with LPS (5 mg/kg, intraperitoneal). Treatments continued for 3 days; on day 4, mice were euthanized for serum cytokine and lung histopathology analysis [5] - SHR hypertension model: Male SHR (12-week-old, n=6/group) were randomized into 2 groups: (1) vehicle (0.5% methylcellulose, oral, daily); (2) Adezmapimod 10 mg/kg (oral, daily). SBP was measured weekly via tail-cuff plethysmography. After 4 weeks, mice were euthanized; hearts were weighed to calculate LVHI (heart weight/body weight × 1000) [4] - Pharmacokinetic (PK) study: Male CD-1 mice (n=3/time point) received Adezmapimod via oral gavage (10 mg/kg, vehicle) or intravenous injection (2 mg/kg, 5% DMSO/95% saline). Blood samples (50 μL) were collected at 0.25, 0.5, 1, 2, 4, 6, 8, 12 hours post-dose. Plasma concentrations were measured via LC-MS/MS; PK parameters were calculated via non-compartmental analysis [2] |
| 药代性质 (ADME/PK) |
1. Solubility & Formulation
o Solubility: SB203580 is highly soluble in DMSO (43 mg/mL or 113.92 mM) but insoluble in water and ethanol. o Formulation: Typically supplied as a powder or in DMSO solution for research use. 2. Absorption & Bioavailability o Oral Administration: In animal studies, SB203580 was administered orally (e.g., dissolved in drinking water at 250 μM) and showed efficacy in disease models. o Intraperitoneal (IP) Injection: Used in mice at 5 mg/kg/day, demonstrating systemic activity. 3. Metabolism & Half-Life o Metabolic Stability: No direct data on metabolic pathways, but storage conditions (-20°C, protected from light) suggest sensitivity to degradation. o In Vivo Efficacy: Effective in inhibiting inflammatory cytokines in mice and rats at doses of 15–60 mg/kg, with IC50 values of 15–25 mg/kg in vivo. 4. Distribution & Protein Binding o Cell Permeability: SB203580 is cell-permeable, allowing intracellular inhibition of p38 MAPK (IC50 = 600 nM in cells). o Tissue Effects: Reduces inflammation in collagen-induced arthritis and endotoxin shock models, indicating broad tissue distribution. 5. Excretion & Clearance o No specific data on excretion pathways, but its effects in animal models suggest moderate clearance (e.g., daily dosing required for sustained activity) Oral bioavailability: In CD-1 mice, Adezmapimod had an oral bioavailability of ~35% (AUC₀₋∞ oral = 12.6 μg·h/mL; AUC₀₋∞ IV = 36.0 μg·h/mL) [2] - Plasma PK: After oral administration (10 mg/kg), Cmax was 2.8 μg/mL (Tmax = 1.2 hours), terminal T₁/₂ = 2.5 hours. After IV administration (2 mg/kg), Cmax = 8.6 μg/mL, T₁/₂ = 2.1 hours [2] - Tissue distribution: In mice (10 mg/kg oral), Adezmapimod had a tumor-to-plasma ratio of 2.9 (MDA-MB-231 xenografts, 2 hours post-dose), moderate liver distribution (liver-to-plasma = 2.3), and low brain penetration (brain-to-plasma = 0.18) [3] - Metabolism: In human liver microsomes, Adezmapimod was primarily metabolized by CYP2D6 (≥55% of total metabolism) and CYP3A4 (~30%). Co-incubation with CYP2D6 inhibitor reduced metabolism by ~65% [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
1. Acute Toxicity & Safety Hazards
• Oral Toxicity: Classified as Acute Toxicity Category 4 (Hazard Statement H302)—harmful if swallowed • Ocular Toxicity: Causes severe eye damage (Category 1, H318); direct contact requires immediate rinsing and medical attention • Handling Precautions: Requires protective gear (gloves, goggles, masks) due to risks of inhalation or skin contact ________________________________________ 2. In Vivo Toxicity Studies Ocular Exposure (Conjunctival Injection) • Study: Subconjunctival injection of 50 μM SB203580 in rats showed no significant corneal toxicity (e.g., intact epithelium, normal stromal arrangement) but caused transient conjunctival anemia that resolved within 24 hours • Conclusion: Low toxicity for short-term ocular applications, though local irritation may occur Systemic Administration • Asthma Model: In smoke-exposed asthmatic rats, SB203580 reduced airway inflammation and improved lung function without reported adverse effects at tested doses • Pancreatitis Model: Inhibited TNF-α and pancreatic acinar cell apoptosis in severe acute pancreatitis rats, suggesting therapeutic potential with no overt toxicity noted ________________________________________ 3. Biochemical & Cellular Toxicity • Off-Target Effects: At high concentrations (>10 μM), SB203580 may non-specifically inhibit kinases like PKB or PDK1 and paradoxically activate ERK/NF-κB pathways • Cell Culture: Cytotoxicity observed in some cell lines (e.g., hepatocytes) at concentrations exceeding its p38 MAPK IC50 (0.3–0.5 μM) ________________________________________ 4. Environmental & Handling Risks • Storage: Stable at -20°C but degrades under heat, moisture, or light exposure • Disposal: Must be incinerated or handled as hazardous waste to avoid environmental contamination Plasma protein binding: Adezmapimod had a plasma protein binding rate of ~94% in human plasma (measured via equilibrium dialysis) [2] - Acute toxicity: In CD-1 mice, single oral doses up to 200 mg/kg did not cause mortality or clinical signs (e.g., lethargy, weight loss). Serum ALT, AST, BUN, and creatinine were within normal ranges 24 hours post-dose [4] - Chronic toxicity: A 28-day repeat-dose study in rats (5–20 mg/kg, oral, daily) showed no significant organ toxicity (liver, kidney, heart) at doses ≤10 mg/kg. At 20 mg/kg, mild renal tubular vacuolation was observed in 2/6 rats [4] - Drug-drug interaction: Adezmapimod did not inhibit CYP1A2, 2C9, 2C19, or 3A4 at clinically relevant concentrations (IC₅₀ >10 μM), indicating low interaction risk [2] |
| 参考文献 | |
| 其他信息 |
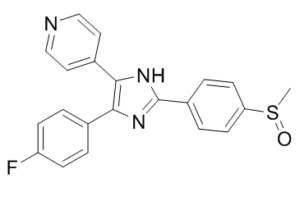
SB 203580 is a member of the class of imidazoles carrying 4-methylsulfinylphenyl, 4-pyridyl and 4-fluorophenyl substituents at positions 2, 4 and 5 respectively. An inhibitor of mitogen-activated protein kinase. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, a Hsp90 inhibitor, a neuroprotective agent, an EC 2.7.11.1 (non-specific serine/threonine protein kinase) inhibitor and a geroprotector. It is a member of imidazoles, a member of monofluorobenzenes, a member of pyridines and a sulfoxide.
4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole has been reported in Annulohypoxylon truncatum, Eleutherococcus divaricatus, and other organisms with data available. \n\nPyridinyl imidazole inhibitors, particularly SB203580, have been widely used to elucidate the roles of p38 mitogen-activated protein (MAP) kinase (p38/HOG/SAPKII) in a wide array of biological systems. Studies by this group and others have shown that SB203580 can have antiproliferative activity on cytokine-activated lymphocytes. However, we recently reported that the antiproliferative effects of SB203580 were unrelated to p38 MAP kinase activity. This present study now shows that SB203580 can inhibit the key cell cycle event of retinoblastoma protein phosphorylation in interleukin-2-stimulated T cells. Studies on the proximal regulator of this event, the phosphatidylinositol 3-kinase/protein kinase B (PKB)(Akt/Rac) kinase pathway, showed that SB203580 blocked the phosphorylation and activation of PKB by inhibiting the PKB kinase, phosphoinositide-dependent protein kinase 1. The concentrations of SB203580 required to block PKB phosphorylation (IC(50) 3-5 microM) are only approximately 10-fold higher than those required to inhibit p38 MAP kinase (IC(50) 0.3-0.5 microM). These data define a new activity for this drug and would suggest that extreme caution should be taken when interpreting data where SB203580 has been used at concentrations above 1-2 microM.[1] \n\nIn the present study we investigated a possible role for the p38 mitogen-activated protein (MAP) kinase pathway in mediating nuclear factor-kappa B (NF-kappaB) transcriptional activity in the erythroleukaemic cell line TF-1. TF-1 cells stimulated with the phosphatase inhibitor okadaic acid (OA) demonstrated enhanced NF-kappaB and GAL4p65-regulated transcriptional activity which was associated with elevated p38 phosphorylation. However, pretreatment with the p38 MAPK specific inhibitor SB203580 (1 microM) or overexpression of kinase-deficient mutants of MKK3 or MKK6 did not affect OA-enhanced NF-kappaB transcriptional potency, as determined in transient transfection assays. In fact, 5 and 10 microM SB203580 enhanced rather than inhibited NF-kappaB-mediated promoter activity by 2 fold, which was independent of phosphorylation of the p65 subunit. The SB203580-mediated increase in NF-kappaB transcriptional activity was associated with enhanced phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK), but not p38 kinase. Overexpression of kinase-deficient mutants belonging to the ERK1/2, JNK, and p38 pathways showed that only dominant-negative Raf-1 abrogated SB203580-enhanced NF-kappaB activity. This would implicate the involvement of the ERK1/2 pathway in the enhancing effects of SB203580 on NF-kappaB-mediated gene transcription. This study demonstrates that the p38 MAP kinase pathway is not involved in the OA-induced activation of NF-kappaB. SB203580 at higher concentrations activates the ERK pathway, which subsequently enhances NF-kappaB transcriptional activity.[2]\n \nSB203580 is a well-known inhibitor of p38 mitogen-activated protein kinase (MAPK). However, it can suppress cell proliferation in a p38 MAPK independent manner. The inhibitory mechanism remains unknown. Here, we showed that SB203580 induced autophagy in human hepatocellular carcinoma (HCC) cells. SB203580 increased GFP-LC3-positive cells with GFP-LC3 dots, induced accumulation of autophagosomes, and elevated the levels of microtubule-associated protein light chain 3 and Beclin 1. It stimulated the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK) and p53, but inhibited the phosphorylation of death-associated protein kinase (DAPK). Inhibition of AMPK, p53, or DAPK attenuated SB203580-induced autophagy. AMPK activation appeared to predate the DAPK signal. The activation of both AMPK and DAPK prompted the phosphorylation of p53 and enhanced Beclin 1 expression. Neither the downregulation of p38 MAPK by its siRNA or chemical inhibitor nor the upregulation of p38 MAPK by p38 MAPK DNA transfection affected B203580-induced autophagy. Collectively, the findings demonstrate a novel function of SB203580 to induce autophagy via activating AMPK and DAPK but independent of p38 MAPK. The induction of autophagy can thus account for the antiproliferative effect of SB203580 in HCC cells.[3]\n \nWe report that SB203580 (SB), a specific inhibitor of p38-MAPK, protects pig myocardium against ischemic injury in an in vivo model. SB was applied by local infusion into the subsequently ischemic myocardium for 60 min before a 60-min period of coronary occlusion followed by 60-min reperfusion (index ischemia). Infarct size was reduced from a control value of 69.3 +/- 2.7% to 36.8 +/- 3.7%. When SB was infused systemically for 10 min before index ischemia, infarct size was reduced to 36.1 +/- 5.6%. We measured the content of phosphorylated p38-MAPK after systemic infusion of SB and Krebs-Henseleit buffer (KHB; negative control) and during the subsequent ischemic period using an antibody that reacts specifically with dual-phosphorylated p38-MAPK (Thr180/ Tyr182). Ischemia with and without SB significantly increased phospho-p38-MAPK, with a maximum reached at 20 min but was less at 30 and 45 min under the influence of the inhibitor. The systemic infusion of SB for 10 min before index ischemia did not significantly change the p38-MAPK activities (compared with vehicle, studied by in-gel phosphorylation) < or =20 min of ischemia, but activities were reduced at 30 and 45 min. Measurements of p38-MAPK activities in situations in which SB was present during in-gel phosphorylation showed significant inhibition of p38-MAPK activities. The systemic infusion of SB significantly inhibited the ischemia-induced phosphorylation of nuclear activating transcription factor 2 (ATF-2). Using a specific ATF-2 antibody, we did not observe significant changes in ATF-2 abundance when nuclear fractions from untreated, KHB-, and SB-treated tissues were compared. We investigated also the effect of local and systemic infusion of SB on the cardioprotection induced by ischemic preconditioning (IP). The infusions (local or systemic) of SB before and during the IP protocol did not influence the infarct size reduction mediated by IP. The observed protection of the myocardium against ischemic damage by SB points to the negative role of the p38-MAPK pathway during ischemia.[4]\n\n \n\nIn summary, we have putatively identified PDK1/PDK2 as targets for the pyridinyl imidazole p38 MAP kinase inhibitor SB203580, which could, at least in part, explain the antiproliferative effect of the drug. The observation that SB203580 can inhibit PI 3-kinase/PDK1/PKB pathway could have major implications for the interpretation of data obtained with this drug. [1]\n \nAlthough activation of ERK and JNK by SB203580 has not been reported before, observations in several studies may suggest the occurrence of this phenomenon. For instance, Schwenger et al. (1998) suggested that TNF-induced p38 kinase activation may exert a negative regulatory influence on the process of NFκB activation by this cytokine in COS-1 cells. At the concentration of 10 μM, SB203580 significantly prevented the ability of the drug sodium salicylate to suppress TNF-induced IκBα degradation. However, in this study it was not ruled out that SB203580 exerted its effect by activating alternate MAPK pathways and thus enhancing the degradation of IκBα.\n\nIn myeloid leukaemic cells, NF-kB expression may exert its clinically unfavourable effect by enhancing the expression of cytokine genes or by inducing the expression of anti-apoptotic genes. Insight into the regulation of NF-κB in these cells may thus lead to new clinical approaches. In the present study we showed that the p38 MAP kinase pathway does not mediate the OA-induced NF-κB activation in the TF-1 haematopoietic cell line. Moreover, SB203580 stimulation may result in adverse effects, since it enhances NF-κB and ERK.[2]\n\n \nNevertheless, our results appear to indicate that SB203580 induces HCC cell autophagy independent of p38 MAPK and caspase-3 via multiple channels, and none of these channels can be 100% responsible for the induction of autophagy. For example, the suppression of AMPK by compound C does not completely inhibit SB203580-induced autophagy, neither does the inhibition of DAPK nor p53. SB203580 is a well-known p38 MAPK inhibitor to block apoptosis induced by various agents. The induction of autophagy by SB203580 may provide us with some novel concepts when dealing with cancer cell death. For example, in some situations, though the apoptosis has been inhibited by SB203580, cell survival may continue to decrease. The autophagy induced by SB203580 should give a reasonable answer to this scientific puzzle, thereby helping the development of more effective treatments for cancers. [3]\n \n\nIn this study and also in our previous experiments, we observed two bands (protein kinases) in the range of 38-45 kDa that use MAPKAPK-2 as a substrate in vitro. We investigated the specificity of the reaction for p38-MAPK by immunoprecipitation with a p38-MAPK polyclonal antibody (C-20). The antibody is specific for p38-MAPK and is not cross-reactive with p38-MAPK-beta. The same antibody was used for detection of p38-MAPK content presented in this study (see Fig. 7A). We found that the antibody reacted very strongly with a 38-kDa protein of molecular mass of (p38-MAPK) and when the immunoprecipitate was tested by in-gel phosphorylation of MAPKAPK-2, we found activity only in the range of 38 kDa. We cannot exclude the possibility that the upper band of 45 kDa represents some isoform of p38-MAPK, but our results show that SB inhibits preferentially the activity of the lower (38-kDa) band. It is known that the p38-MAPK exists in at least six isoforms (two alternative spliced isoforms α and β and isoforms τ and δ). These p38-MAPKs differ in their sensitivity to stimulation, inhibitor sensitivity, and also substrate specificity. We cannot exclude the possibility that more than one isoform of p38-MAPK is activated during myocardial ischemia. However, PC12 cells showed a selective activation of p38-MAPK-α and p38-MAPK-γ by hypoxia. Hypoxia had no effect on the activity of the β and δ isoforms. Our results obtained with SB in vitro (phosphorylation step of in-gel assay; Fig. 8A and C) show that SB fully inhibited the ischemia-induced p38-MAPK activity. It has been described that the γ and δ isoforms are resistant to inhibition by SB/SB203580. This would suggest that these two p38-MAPK isoforms (γ and δ) are not involved in the effects of SB during ischemia and in mechanisms leading to ischemic death.\n\nIn conclusion, we provide detailed information about the detrimental effect of p38-MAPK activation during ischemia, which can be inhibited by SB. We have provided further evidence for our hypothesis that ischemia/reperfusion activates different signaling cascades with opposing effects on survival, of which the ERKs and the SAPK/JNKs favor survival, and the p38-MAPKs accelerate cell death. The development of future treatment strategies for ischemic syndromes may find these observations useful. [4]\n\n \nCollectively, p38 MAPK inhibitor may dampen the autoimmune attack in important organs and improve renal function. Furthermore, serum levels of ALT and AST were not increased in SB203580 treated MRL/lpr mice and the survival of SB203580 treated MRL/lpr mice was similar to untreated MRL/lpr mice during the experimental period, indicating no obvious adverse effects on lifespan and liver function of SB203580. In spite of the efficacy of SB203580 observed, the inhibition of p38 MAPK is not sufficient to completely prevent organ injuries due to the findings that almost all the pathological profiles analyzed in our study were not able to be recovered by SB203580 in MRL/lpr mice compared with negative control C57BL/6 mice. Further studies with a larger number of animals and other strains of lupus-prone animal models will be needed to confirm the efficacy and mechanism of the action of SB203580, as to provide its potential clinical application for human disease.\nThough many different p38 MAPK inhibitors have been undergoing clinical trials, none of them has yet passed accreditation successfully for the treatment of autoimmune diseases on account of safety concerns associated with potential cross reactivities with other kinases. And unfortunately adverse effects on the central nervous system and liver have been observed in phase II clinical trials of VX745 and BIRB796. But up till now, there is no report concerning the side effect of SB203580. Therefore, further investigation of SB203580 is required to verify its safety in many other systems. Evaluation of the life span and clinical improvement after treatment being discontinued is also needed. [5] Mechanism of action: Adezmapimod is a reversible, ATP-competitive inhibitor of p38α/β. It binds to the ATP-binding pocket of p38, preventing ATP coordination and subsequent phosphorylation of downstream substrates (e.g., MK2, HSP27) [1] - Research applications: It is widely used as a tool compound to study p38-mediated pathways in inflammation, cancer, and cardiovascular disease. It entered Phase II clinical trials for rheumatoid arthritis but was discontinued due to modest efficacy [5, 4] - Selectivity note: Unlike non-selective p38 inhibitors, it shows minimal activity against JNK/ERK, reducing off-target effects in inflammatory and cardiovascular models [1, 2] |
| 分子式 |
C21H16FN3OS
|
|---|---|
| 分子量 |
377.43
|
| 精确质量 |
377.099
|
| 元素分析 |
C, 66.83; H, 4.27; F, 5.03; N, 11.13; O, 4.24; S, 8.49
|
| CAS号 |
152121-47-6
|
| 相关CAS号 |
Adezmapimod hydrochloride;869185-85-3
|
| PubChem CID |
176155
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
615.6±55.0 °C at 760 mmHg
|
| 熔点 |
249 - 250ºC
|
| 闪点 |
326.1±31.5 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.715
|
| LogP |
4.1
|
| tPSA |
77.85
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
500
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(C([H])([H])[H])(C1C([H])=C([H])C(=C([H])C=1[H])C1=NC(C2C([H])=C([H])C(=C([H])C=2[H])F)=C(C2C([H])=C([H])N=C([H])C=2[H])N1[H])=O
|
| InChi Key |
CDMGBJANTYXAIV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25)
|
| 化学名 |
4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine;hydrochloride
|
| 别名 |
RWJ 64809; PB 203580; Adezmapimod; 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole; 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine; 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine; RWJ64809; SB203580; SB203580; SB 203580; RWJ-64809; PB-203580; PB203580
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.62 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2 mg/mL (5.30 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 例如,若需制备1 mL的工作液,可将 100 μL 20.0 mg/mL 澄清的 DMSO 储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL 生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 2 mg/mL (5.30 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 配方 4 中的溶解度: 2 mg/mL (5.30 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 例如,若需制备1 mL的工作液,您可以将 100 μL 20.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 配方 5 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O: 5mg/mL 配方 6 中的溶解度: 16.67 mg/mL (44.17 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6495 mL | 13.2475 mL | 26.4950 mL | |
| 5 mM | 0.5299 mL | 2.6495 mL | 5.2990 mL | |
| 10 mM | 0.2649 mL | 1.3247 mL | 2.6495 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
 |
 |