| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
MEK2; MEK1 (IC50 = 14 nM); MEK (IC50 = 12 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Selumetinib 不具有 ATP 竞争性,可抑制 ERK1/2 磷酸化,IC50 水平低于 40 nM。通过抑制 ERK1/2 和 p90RSK 磷酸化,以及提高 caspase-3 和 caspase-7 裂解和裂解聚 (ADP) 核糖聚合酶,AZD6244 还可以减缓原发性 HCC 细胞的生长。 p38、c-Jun-NH2-激酶、磷脂酰肌醇 3-激酶和 MEK5/ERK5 通路不受 AZD6244 的显着影响。 [1] 乳腺癌细胞系中的 Raf 突变和 NSCLC 细胞系中的 Ras 突变均对 AZD6244 有反应。 [2]
|
| 体内研究 (In Vivo) |
Selumetinib 显着抑制 2-1318、5-1318、26-1004 和 4-1318 异种移植物中 ERK1/2 的磷酸化,并通过激活 caspase 途径诱导原代 2-1318 细胞凋亡。在 100 mg/kg 的剂量下,AZD6244 可以减缓 HT-29 异种移植物(一种具有 B-Raf 突变的结直肠肿瘤模型)中肿瘤的生长;这种肿瘤生长抑制作用优于吉西他滨。细胞凋亡和细胞周期调节因子(如细胞周期蛋白 D1、Cdc-2、CDK2 和 4、细胞周期蛋白 B1 和 c-Myc)的下调与细胞凋亡增加相关,这就是为什么 AZD6244 可以在不存在的情况下抑制 HCC 异种移植肿瘤的生长。这些因素。
|
| 酶活实验 |
MEK1。[3]
nh2末端六组氨酸标记,组成活性MEK1 (S218D, S222D ΔR4F;参考文献18)在杆状病毒感染的Hi5昆虫细胞中表达,并通过固定化金属亲和层析、离子交换和凝胶过滤纯化。通过测定[γ-33P]ATP中[γ-33P]磷酸在ERK2上的掺入量来评估MEK1的活性。在96孔聚丙烯板上,用25 mmol/L HEPES (pH 7.4)、10 mmol/L MgCl2、5 mmol/L β-甘油磷酸、100 μmol/L正钒酸钠、5 mmol/L DTT、5 nmol/L MEK1、1 μmol/L ERK2和0 ~ 80 nmol/L化合物(终浓度为1% DMSO)组成的培养液(100 μL)进行测定。加入10 μmol/L ATP (0.5 μC k[γ-33P]ATP/孔),室温孵育45 min,加入等量的25%三氯乙酸停止反应,沉淀蛋白质。沉淀的蛋白质被捕获在玻璃纤维B滤板上,多余的标记ATP用0.5%磷酸洗掉,并在液体闪烁计数器中计算放射性。通过改变反应混合物中ATP的量来确定ATP依赖性。使用SigmaPlot对数据进行全局拟合。非竞争性抑制的计算公式如下:v = [Vmax × S / (1 + I / Ki)] / (Km + S)。[3] ERK2。[3] 为了测量ERK2的抑制作用,首先用MEK1激活ERK2的激酶活性。野生型(WT) ERK2含有nh2末端六组氨酸标签,在大肠杆菌中过表达,并通过固定化金属亲和层析、离子交换和凝胶过滤纯化。为了激活WT ERK2,将2 mg WT ERK2与17 μg组成活性MEK1混合在4 mL 25 mmol/L HEPES (pH 7.5)中,其中含有1 mmol/L ATP。反应混合物在室温下孵育40分钟,并通过质谱法确定了两种磷酸盐的加入。活化的WT ERK2通过离子交换进一步纯化。使用10 nmol/L活化的ERK2,按照组成活性MEK的描述检测ERK2活性。底物为髓鞘碱性蛋白,浓度为1 μmol/ l。 [3] 使用抗MEK1抗体免疫沉淀MEK1分子。在以MBP为终点的偶联实验中,当重组ERK1被免疫分离的MEK1激活时,计算MEK激酶活性。在暴露于x射线胶片之前,磷酸化的MBP在14% SDS-PAGE凝胶上分解并真空干燥。 |
| 细胞实验 |
细胞活力与细胞增殖[1]
原代HCC细胞以每孔2.0 × 104的密度在生长培养基中接种。在培养基中培养48 h后,用MEM冲洗细胞单层2次。用不同浓度的AZD6244(0、0.5、1.0、2.0、3.0和4.0 μmol/L)处理细胞24或48 h,采用3-(4,5-二甲基噻唑-2y1)-2,5-二苯基溴化四唑(MTT)法测定细胞活力(32)。使用制造商描述的溴脱氧尿苷试剂盒(罗氏)检测细胞增殖。实验至少重复3次,数据用mean±SE表示[1] 。 细胞凋亡检测[1] 在8室载玻片中培养HCC原代细胞,在SRF培养基中分别用0、0.5、1.0、2.0、3.0和4.0 μmol/L AZD6244处理24 h,用含4%福尔马林溶液的PBS在室温下固定细胞1 h,并用PBS洗涤。凋亡通过末端脱氧核苷酸转移酶介导的dUTP镍端标记(TUNEL)检测,使用制造商描述的原位细胞死亡检测试剂盒(罗氏)。然后在配备FITC滤镜的荧光显微镜下观察凋亡细胞。标记指数通过计算每个区域500个细胞中的阳性细胞数得到。以百分数表示。[1] 在2.0 × 104的密度下,细胞被播种。培养48小时后,细胞经过两次培养基冲洗。AZD6244用于在不同浓度下治疗细胞24或48小时。MTT法使用3-(4,5-二甲基噻唑-2y1)-2,5-二苯基溴化四唑来测量细胞的活力。在溴脱氧尿苷试剂盒的帮助下,测量细胞增殖。 |
| 动物实验 |
HCC xenografts in mice homozygous for the SCID (severe combined immunodeficiency) mutation
50 or 100mg/kg Administered via p.o. o investigate the effects of AZD6244 on HCC xenografts, AZD6244 was suspended in water at an appropriate concentration. Mice bearing HCC xenografts were p.o. given, twice a day, with either 100 μL of water (n = 12) or 50 mg (n = 12) or 100 mg (n = 12) of AZD6244 per kilogram of body weight for 21 days, starting from day 7 after tumor implantation. Growth of established tumor xenografts was monitored at least twice weekly by Vernier caliper measurement of the length (a) and width (b) of the tumor. Tumor volume was calculated as (a × b2)/2. Animals were sacrificed 3 h after the last dose of ADZ6244, and body and tumor weights were recorded, with the tumors harvested for analysis.[1] To study the effects of AZD6244 on caspase-3 activation and MEK1/2 phosphorylation, mice bearing HCC tumors (∼800 mm3) were treated with vehicle (n = 4) or 50 mg of AZD6244 per kilogram of body weight (n = 4) for 3 days as described above. Animals were sacrificed 3 h after the last dose, and tumors were harvested and frozen in liquid nitrogen for later analysis. Part of the tumor harvest was fixed in neutral buffer containing 10% formalin for immunohistochemistry.[1] HT-29 human colon carcinoma or BxPC3 human pancreatic tumor fragments were implanted s.c. in the flank of nude mice and allowed to grow to 100 to 150 mg. Mice (n = 10 per group) were randomized to treatment groups to receive vehicle (10 mL/kg and 10% ethanol/10% cremophor EL/80% D5W) or AZD6244/ARRY-142886 (10, 25, 50, or 100 mg/kg, oral, BID) on days 1 to 21. Tumors [(W2 × L) / L] were measured twice weekly. [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Based on several studies investigating selumetinib at various doses in both pediatric and adult populations, the Tmax generally ranges between 1- 1.5 hours. In healthy adults, the mean absolute oral bioavailability was reported to be 62%. Selumetinib should be administered on an empty stomach since food significantly decreases serum concentrations of the drug. Approximately 59% of selumetinib is eliminated in the feces, while 33% is eliminated in the urine. The mean apparent volume of distribution of selumetinib at steady state in pediatric patients ranged from 78 L to 171 L. A study in healthy adult males found a mean apparent volume of distribution of 146 L. Another study observing the pharmacokinetic effects of various selumetinib doses and regimens in select Japanese patients found that the apparent volume of distribution values at steady-state ranged from 73.2 - 148.1 L. The clearance of selumetinib in pediatric patients is 8.8 L/hr. A study in healthy adult males found a clearance value of 15.7 L/hr. Another study observing the pharmacokinetic effects of various selumetinib doses and regimens in select Japanese patients found clearance values that ranged from 9.2 - 15.9 L/hr. Metabolism / Metabolites Selumetinib is heavily metabolized in the liver and the proposed metabolic pathway is as follows: Hydrolysis of selumetinib’s amide functional group produces M15 (AZ13326637), which contains a carboxylic acid. Elimination of the ethanediol moiety from the parent compound results in the formation of the primary amide M14 (AZ12791138) metabolite. Amide hydrolysis transforms M14 into M15, glucuronidation and further oxidation of M14 leads to M2, M6 and M1, and N-demethylation of M14 produces M12. The amide glucuronide (M2) is thought to be the major circulating metabolite. Demethylation of selumetinib produces the pharmacologically active M8 (AZ12442942), and further oxidation of M8 leads to M11. Glucuronidation of M8 produces M3 or M5, and elimination of the ethanediol moiety from M8 results in a primary amide, producing M12. Although the N-demethylated metabolite (M8) accounts for <10% of the circulating metabolites, it is responsible for approximately 21-35% of any observed pharmacological activity. Ribose conjugation transforms M12 into M9, while oxidation of M12 leads to M10 and M13 metabolites. Glucuronidation of M10 produces M1. Direct glucuronidation of selumetinib produces M4 or M7, which can both eventually transform into M3 and M5 metabolites. Biological Half-Life Selumetinib is characterized by a short half-life. The elimination half-life associated with a dose of 25 mg/m2 in pediatric patients is 6.2 hours. In a study observing the pharmacokinetic effects of various selumetinib regimens in select Japanese patients, the half-life ranged from 9.2- 10.6 hours. In other studies where selumetinib 75 mg is administered twice daily, the half-life is reported to be approximately 13 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure clinical trials conducted in children and adults with neurofibromatosis, serum aminotransferase elevations occurred in 35% of treated subject but rose to above 5 times the upper limit of normal (ULN) in only 4%. However, there were no liver related serious adverse events and no patient had a concurrent elevation in serum aminotransferase and bilirubin levels. The ALT elevations were typically mild and transient and usually resolved even without dose adjustment. There were no instances of clinically apparent liver injury attributed to selumetinib. Since approval and more wide scale availability of selumetinib, there have been no published reports of clinically apparent drug induced liver injury associated with its use in neurofibromatosis, although clinical experience with the drug, particularly with long term therapy, has been limited. However, in studies of experimental therapy with somewhat higher doses of selumetinib in patients with advanced, refractory cancers, liver test abnormalities were common and sometimes graded as severe (ALT above 20 times ULN) and requiring drug discontinuation. Thus, in patients with neurofibromatosis and use of recommended doses of selumetinib, clinically apparent liver injury is rare if it occurs at all. At higher doses, however, selumetinib has been associated with a very high rate of serum enzyme elevations, many of which were in the range suggestive of severe injury. Likelihood score: E* (suspected but unproven rare cause of clinically apparent liver injury). Protein Binding Separate studies investigating selumetinib protein binding found that 96% of selumetinib was bound to serum albumin, while <35% was bound to ɑ-1 acid glycoprotein. Overall, approximately 98.4% of selumetinib is plasma protein bound. |
| 参考文献 |
[1]. Mol Cancer Ther . 2007 Jan;6(1):138-46. [2]. Mol Cancer Ther . 2010 Jul;9(7):1985-94. [3]. Clin Cancer Res . 2007 Mar 1;13(5):1576-83. [4]. Mol Cancer Ther . 2007 Sep;6(9):2468-76. [5]. Mol Cancer Ther . 2007 Aug;6(8):2209-19. [6]. Clin Cancer Res . 2012 Feb 15;18(4):1051-62. [7]. Int J Oncol . 2012 Aug;41(2):712-20. [8]. J Clin Endocrinol Metab . 2008 Jun;93(6):2194-201. [9]. Proc Natl Acad Sci U S A . 2009 Dec 1;106(48):20411-6. [10]. Cancer Res . 2008 Aug 1;68(15):6145-53. [11]. J Hepatol . 2010 Jan;52(1):79-87. |
| 其他信息 |
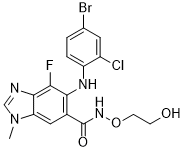
Selumetinib is a member of the class of benzimidazoles that is 1-methyl-1H-benzimidazole which is substituted at positions 4, 5, and 6 by fluorine, (4-bromo-2-chlorophenyl)amino, and N-(2-hydroxyethoxy)aminocarbonyl groups, respectively. It is a MEK1 and MEK2 inhibitor. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an antineoplastic agent and an anticoronaviral agent. It is a member of benzimidazoles, a hydroxamic acid ester, a member of monochlorobenzenes, a member of bromobenzenes, an organofluorine compound and a secondary amino compound.
Activation of the Raf-MEK-ERK signalling pathway is known to be implemented in several types of malignancies; thus, mitogen-activated protein kinase kinase (MEK) inhibitors such as selumetinib are important tools that can target the problematic overactivity of this pathway. Results from clinical trials investigating earlier developed MEK inhibitors were underwhelming. However, selumetinib demonstrated impressive efficacy and tolerability in Phase I trials, leading to its continued investigation for the treatment of various types of tumours in Phase II trials. Currently, the novel MEK 1 / 2 inhibitor, selumetinib, is approved solely for the treatment of Neurofibromatosis type 1 (NF-1) in a limited age group. NF-1 is considered rare, with an estimated incidence of 1/3000 individuals. It is a genetic, autosomal dominant condition resulting from mutations of the NF1 gene, which can lead to various complications, including the development of multiple tumours in the nervous system. Some patients with this disorder develop plexiform neurofibromas (PN); however, this is considered relatively uncommon compared to other variants of NF-1. Luckily, the use of selumetinib in patients with NF-1 have shown efficacy in shrinking associated tumours and is linked to other positive clinical outcomes. Selumetinib was approved by the FDA on April 10, 2020. It was later approved by Health Canada on August 23, 2022. Selumetinib is a Kinase Inhibitor. The mechanism of action of selumetinib is as a Mitogen-Activated Protein Kinase Kinase 1 Inhibitor, and Mitogen-Activated Protein Kinase Kinase 2 Inhibitor. Selumetinib is an oral, small molecule inhibitor of the mitogen activated protein kinase 1 and 2 (MEK1/2) that is used to treat symptomatic, refractory fibromas in neurofibromatosis type 1. Selumetinib is associated with transient and usually mild elevations in serum aminotransferase levels during therapy, but has not been linked to cases of clinically apparent acute liver injury. Selumetinib is an orally active, small molecule with potential antineoplastic activity. Selumetinib is an ATP-independent inhibitor of mitogen-activated protein kinase kinase (MEK or MAPK/ERK kinase) 1 and 2. MEK 1 and 2 are dual specificity kinases that are essential mediators in the activation of the RAS/RAF/MEK/ERK pathway, are often upregulated in various cancer cells, and are drivers of diverse cellular responses, including proliferation. Inhibition of both MEK1 and 2 by selumetinib prevents the activation of MEK1/2 dependent effector proteins and transcription factors, thereby leading to an inhibition of cellular proliferation in various cancers. See also: Selumetinib Sulfate (has salt form). Drug Indication Selumetinib is indicated for the treatment of neurofibromatosis type 1 (NF1) in patients two years and older who have symptomatic, inoperable plexiform neurofibromas (PN). Koselugo as monotherapy is indicated for the treatment of symptomatic, inoperable plexiform neurofibromas (PN) in paediatric patients with neurofibromatosis type 1 (NF1) aged 3 years and above Treatment of melanoma, Treatment of neurofibromatosis type 1, Treatment of thyroid cancer Mechanism of Action The Ras-Raf-MEK-ERK signaling cascade is known to be activated in several types of cancer, and regulates the transcription of proteins involved in apoptosis. In addition, studies have shown that mutations of the Raf component of the pathway can contribute to chemotherapy drug resistance. Ras as well as several kinases and phosphatases are responsible for regulating the Raf-MEK-ERK pathway. Often in cancers, Ras (a G-protein coupled receptor) is deregulated, allowing downstream signalling to proceed unchecked. Through several complex steps, Raf phosphorylates and activates MEK, which then phosphorylates and activates ERK. ERK is then able to exert its effects on several downstream targets. As such, therapies inhibiting upstream components of this pathway have become attractive targets for cancer treatment. Selumetinib exerts its effects by selectively inhibiting MEK1 and MEK2 which can effectively blunt the pleiotropic effects of the Ras-Raf-MEK-ERK cascade. By inhibiting this oncogenic pathway, selumetinib reduces cell proliferation, and promotes pro-apoptotic signal transduction. |
| 分子式 |
C17H15BRCLFN4O3
|
|---|---|
| 分子量 |
457.68
|
| 精确质量 |
456
|
| 元素分析 |
C, 44.61; H, 3.30; Br, 17.46; Cl, 7.75; F, 4.15; N, 12.24; O, 10.49
|
| CAS号 |
606143-52-6
|
| 相关CAS号 |
Selumetinib sulfate;943332-08-9
|
| PubChem CID |
10127622
|
| 外观&性状 |
white solid powder
|
| 密度 |
1.7±0.1 g/cm3
|
| 折射率 |
1.672
|
| LogP |
5.55
|
| tPSA |
88.41
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
523
|
| 定义原子立体中心数目 |
0
|
| SMILES |
BrC1C([H])=C([H])C(=C(C=1[H])Cl)N([H])C1=C(C2=C(C([H])=C1C(N([H])OC([H])([H])C([H])([H])O[H])=O)N(C([H])([H])[H])C([H])=N2)F
|
| InChi Key |
CYOHGALHFOKKQC-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26)
|
| 化学名 |
6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide
|
| 别名 |
selumetinib; ARRY-142886; AZD6244; ARRY142886; ARRY 142886; AZD-6244; AZD 6244; ARRY886; ARRY-886; ARRY 886; 5-[(4-BROMO-2-CHLOROPHENYL)AMINO]-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZIMIDAZOLE-6-CARBOXAMIDE
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1 mg/mL (2.18 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 10.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1 mg/mL (2.18 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 10.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1 mg/mL (2.18 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4% DMSO+30% PEG 300+5% Tween 80+ddH2O: 5mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1849 mL | 10.9247 mL | 21.8493 mL | |
| 5 mM | 0.4370 mL | 2.1849 mL | 4.3699 mL | |
| 10 mM | 0.2185 mL | 1.0925 mL | 2.1849 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03326310 | Recruiting | Drug: Azacitidine Drug: Selumetinib |
Chronic Myeloid Leukemia Myelofibroses |
University of Chicago | September 4, 2018 | Phase 1 |
| NCT04924608 | Active Recruiting |
Drug: Selumetinib Other: Placebo |
Neurofibromatosis 1 Plexiform Neurofibroma (PN) |
AstraZeneca | November 19, 2021 | Phase 3 |
| NCT04590235 | Active Recruiting |
Drug: Selumetinib | Neurofibromatosis 1 Neurofibroma Plexiform |
AstraZeneca | December 16, 2020 | Phase 1 |
| NCT05101148 | Active Recruiting |
Drug: Selumetinib | Neurofibromatosis Type 1 | AstraZeneca | July 21, 2021 | Phase 1 |
| NCT03095248 | Recruiting | Drug: Selumetinib | Glioma Meningioma |
Children's Hospital Medical Center, Cincinnati |
May 8, 2017 | Phase 2 |
Structure of ARRY-142886 and its ability to inhibit enzymatic MEK1 activity.Clin Cancer Res.2007 Mar 1;13(5):1576-83. |
Inhibition of tumor growth and decreased tumor phospho-ERK1/2 levels in a mouse HT-29 xenograft model.
Inhibition of basal and induced ERK1/2 phosphorylation in human cancer cell lines and PBMCs.Clin Cancer Res.2007 Mar 1;13(5):1576-83. |
Tumor growth inhibition in the mouse BxPC3 xenograft model.Clin Cancer Res.2007 Mar 1;13(5):1576-83. |
 |
 |