| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Flk-1 (IC50 = 1.23 μM)
Semaxanib (SU5416) is a potent and selective inhibitor of the vascular endothelial growth factor receptor-2 (VEGFR-2/Flk-1/KDR), which inhibits tyrosine kinase activity and downstream signaling. [1] It also inhibits the stem cell factor receptor tyrosine kinase c-Kit, often expressed in acute myelogenous leukemia cells. [8] Semaxanib (SU-5416) selectively inhibits vascular endothelial growth factor receptor 2 (VEGFR2/Flk-1/KDR) tyrosine kinase (IC₅₀ = 140 nM for recombinant VEGFR2; IC₅₀ = 250 nM for VEGFR2 phosphorylation in HUVECs) [1] Semaxanib (SU-5416) shows weak inhibitory activity against PDGFRβ (IC₅₀ = 3.0 μM) and c-Kit (IC₅₀ = 5.0 μM), with no significant effect on EGFR, FGFR, or Abl (IC₅₀ > 10 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
Semaxanib 抑制过表达 Flk-1 的 NIH 3T3 细胞中 Flk-1 受体的 VEGF 依赖性磷酸化,IC50 为 1.04 μM。 Semaxanib 抑制 NIH 3T3 细胞中 PDGF 依赖性自磷酸化,IC50 为 20.3 μM。 Semaxanib 以剂量依赖性方式抑制 VEGF 和 FGF 驱动的有丝分裂,IC50 分别为 0.04 和 50 μM。 Semaxanib治疗对C6神经胶质瘤、Calu 6肺癌、A375黑色素瘤、A431表皮样癌和SF767T神经胶质瘤细胞的体外生长没有影响(所有IC50 > 20 μM)。激酶测定:将 3T3 Flk-1 细胞的溶解膜添加到已预先涂有可识别 Flk-1 的单克隆抗体的聚苯乙烯 ELISA 板上。在 4℃ 下与裂解物孵育过夜后,将 SU5416 的系列稀释液添加到免疫定位受体中。为了诱导受体的自身磷酸化,将不同浓度的 ATP 添加到含有系列稀释的 SU5416 溶液的 ELISA 板孔中。自磷酸化在室温下进行 60 分钟,然后用 EDTA 终止。通过将免疫定位受体与针对磷酸酪氨酸的生物素化单克隆抗体一起孵育来确定各个孔中 Flk-1 受体上存在的磷酸酪氨酸的量。除去未结合的抗磷酸酪氨酸抗体后,将亲和素缀合的辣根过酸酶 H 添加到孔中。将稳定形式的 3,3 9,5,5 9-四甲基联苯胺二盐酸盐和 H2O2 添加到孔中。测定的颜色读数允许显色 30 分钟,并用 H2SO4 终止反应。细胞测定:将 HUVEC 接种于 96 孔平底板(1×104 个细胞/100 μL/孔)中,加入含 0.5% 热灭活 FBS 的 F-12K 培养基中,37 ℃ 培养 24 h,使细胞静止。 。然后添加在含有 1% DMSO 的培养基中制备的化合物的连续稀释液 2 小时,然后添加促有丝分裂浓度的 VEGF(5 ng/mL 或 20 ng/mL)或酸性成纤维细胞生长因子(0.25-5 ng/mL)在媒体中。测定中 DMSO 的终浓度为 0.25%。 24小时后,添加[3H]胸苷(1μCi/孔)或BrdUrd,并将细胞单层再孵育24小时。分别使用液体闪烁计数器或 BrdUrd ELISA 对细胞摄取的 [3H] 胸苷或 BrdUrd 进行定量。
塞马西尼(SU-5416)剂量依赖性抑制血管内皮生长因子(VEGF)诱导的人脐静脉内皮细胞(HUVECs)增殖,IC₅₀为0.3μM。1μM时,可抑制HUVECs迁移约80%、管腔形成约85%,并阻断VEGF介导的VEGFR2磷酸化及下游Akt/ERK1/2信号通路[1] 塞马西尼(SU-5416)抑制血小板衍生生长因子-BB(PDGF-BB)诱导的血管平滑肌细胞(VSMCs)增殖,IC₅₀为2.5μM。1μM时可减少A549肺癌细胞中血管生成因子(VEGF、bFGF)的分泌约40%[2] 在黑色素瘤细胞系(A375、SK-MEL-28)中,塞马西尼(SU-5416)在浓度高达10μM时无直接抗增殖作用,但与沙利度胺联合使用时,可增强其细胞毒性约30%[5] |
| 体内研究 (In Vivo) |
Semaxanib 剂量相关地抑制体内 A375 肿瘤的生长。每日腹膜内施用 Semaxanib 的 DMSO 中的 SU5416 时,观察到皮下肿瘤生长抑制 >85%,且没有可测量的毒性。 Semaxanib 显示出广谱抗肿瘤活性。 SU5416 显着抑制所测试的 10 个肿瘤系中的 8 个(A431、Calu-6、C6、LNCAP、EPH4-VEGF、3T3HER2、488G2M2 和 SF763T 细胞)的皮下生长,平均死亡率为 2.5%。 Semaxanib(25 mg/kg/天)显示出有效的抗血管生成活性,导致肿瘤微血管系统的总血管密度和功能血管密度显着降低。
Semaxanib/SU5416 +缺氧(SuHx)大鼠模型是重度肺动脉高压常用模型。虽然已知缺氧暴露可以被另一种类型的打击(例如,卵清蛋白敏化)所取代,但尚不清楚肺血流量异常(PBF)是否可以取代缺氧暴露,这种异常一直被认为会引起肺血管的病理改变。本研究研究 Semaxanib/SU5416联合肺切除术(PNx)诱导对侧肺PBF异常是否足以诱导大鼠严重肺动脉高压(PAH)。Sprague Dawley大鼠采用SuPNx方案(SU5416 +联合左侧全肺切除术)或标准SuHx方案,并在启动后第2周和第6周进行模型比较。SuHx和SuPNx模型均表现出广泛的闭塞性血管重构,导致第6周右心室收缩压升高。两种模型的肺血管中均观察到类似的炎症反应,内皮细胞增殖和凋亡增加。本研究描述了SuPNx模型,该模型在6周时具有严重的多环芳烃,可以作为SuHx模型的替代方案。我们的研究以及先前对肺动脉高压实验模型的研究表明,PAH的典型组织病理学表现,包括闭塞性病变、炎症、细胞更新增加和持续的细胞凋亡,代表了一种疾病的最终共同途径,这种疾病可以作为肺脉管系统受到各种损害的结果而发展。[3] 最近报道了肺动脉高压(PAH)期间肺血管和右心室细胞显著的糖酵解移位。由于该疾病的任何变体的晚期并发症和破坏性过程,因此非常需要重现PAH潜在代谢重编程的动物模型。我们的目的是通过代谢组学分析和分子成像原位研究PAH小鼠模型肺和右心室的代谢重编程。PAH是由慢性缺氧暴露加血管内皮生长因子受体抑制剂Semaxanib/SU5416)治疗引起的。采用磁共振波谱法对肺和右心室样本进行分析。采用正电子发射断层扫描研究体内能量代谢。我们的研究结果表明,肺样本的代谢组学分析清楚地确定了糖酵解途径的显著改变。我们还证实了谷氨酰胺代谢的上调和脂质代谢的改变。此外,我们还发现了肺组织中甘氨酸和胆碱代谢的改变。在右心室样本中也证实了代谢重编程。乳酸和丙氨酸,糖酵解氧化的终点,在多环芳烃小鼠中发现浓度增加。谷氨酰胺和牛磺酸浓度与特定心室肥厚特征相关。我们证明了人类PAH的大多数代谢特征在缺氧加Semaxanib/SU5416小鼠模型中被检测到,它可能成为测试这种严重疾病的新靶向治疗的有价值的工具。[4] 塞马西尼(SU-5416)抑制裸鼠体内多种异种移植瘤的生长和血管生成,包括结直肠癌(Colo205)、乳腺癌(MDA-MB-231)和肺癌(A549)。腹腔注射20mg/kg/天,持续21天,肿瘤体积减少55-70%,瘤内微血管密度(CD31阳性)降低60-75%[1] 通过活体多荧光视频显微镜观察,塞马西尼(SU-5416)(10mg/kg/天,腹腔注射,持续14天)可破坏Lewis肺癌异种移植瘤的微循环,降低血管通透性并诱导血管退化[2] 塞马西尼(SU-5416)(20mg/kg/周,皮下注射)与肺切除术联合使用,可诱导大鼠发生严重肺动脉高压,平均肺动脉压较假手术对照组升高2.5倍[3] 在转移性黑色素瘤患者的II期临床研究中,塞马西尼(SU-5416)(65mg/m²,每周两次,静脉注射)联合沙利度胺的疾病控制率(稳定疾病)为28%,无部分缓解或完全缓解病例[5] 在晚期实体瘤患者的I期研究中,塞马西尼(SU-5416)(40-80mg/m²,每周一次,静脉注射)联合顺铂和伊立替康的毒性可控,但抗肿瘤活性有限,15%的患者达到稳定疾病[6] |
| 酶活实验 |
Semaxanib (SU5416) 通过竞争性结合ATP位点抑制VEGFR-2酪氨酸激酶活性,激酶抑制实验显示其对VEGFR-2具有高度选择性。
[1]
然后用 3T3 Flk-1 细胞的可溶性膜填充预涂有 Flk-1 特异性单克隆抗体的聚苯乙烯 ELISA 板。在 4°C 下与裂解物一起孵育过夜后,将 SU5416 的系列稀释液添加到免疫定位受体中。含有连续稀释的 SU5416 溶液的 ELISA 板孔中充满了不同浓度的 ATP,以引起受体的自身磷酸化。室温下 60 分钟后,使用 EDTA 终止自磷酸化过程。将免疫定位受体与针对磷酸酪氨酸的生物素化单克隆抗体一起孵育,以测量每个孔中 Flk-1 受体上磷酸酪氨酸的量。提取未结合的抗磷酸酪氨酸抗体后,将与亲和素缀合的智人添加到孔中。将稳定形式的三、三、五、九四甲基联苯胺二盐酸盐与H2O2一起添加到每个孔中。 H2SO4 用于在 30 分钟后停止反应,让测定的颜色读数继续进行。 将重组人VEGFR2(Flk-1)激酶结构域与ATP及特异性多肽底物在系列稀释的塞马西尼(SU-5416)存在下孵育,反应在37°C下进行60分钟,采用放射免疫法检测磷酸化底物。通过与溶媒对照组的放射性对比计算抑制率,从量效曲线中得出IC₅₀值[1] 采用相同方案检测塞马西尼(SU-5416)对重组PDGFRβ、c-Kit、EGFR和FGFR激酶的抑制活性,反应条件保持一致,通过确定IC₅₀值证实其对VEGFR2的选择性靶向[1] |
| 细胞实验 |
在内皮细胞培养实验中,Semaxanib (SU5416) 可抑制VEGF诱导的细胞增殖(BrdU掺入法)和迁移(Transwell实验)。Western blot分析证实其能有效抑制VEGFR-2磷酸化。
[1]
在HepG2和TAMH细胞中,细胞毒性实验显示其具有剂量依赖性的抑制活性,IC50值在微摩尔级别。 [8] 将 HUVEC 接种到 96 孔平底板(1×10 4 细胞/100 μL/孔)中,并在含有以下成分的 F-12K 培养基中于 37 °C 培养 24 小时以使细胞静止0.5% 热灭活胎牛血清。添加在含有 1% DMSO 的培养基中制备的化合物的连续稀释液两小时后,然后向培养基中补充促有丝分裂浓度的酸性成纤维细胞生长因子 (0.5–5 ng/mL) 或 VEGF(5 ng/mL 或 20 ng) /毫升)。在测定中,最终DMSO浓度为0.25%。一整天后,添加 BrdUrd 或 [ 3 H]胸苷(1 μCi/孔),将单层细胞再培养 24 小时。可以使用液体闪烁计数器或 BrdUrd ELISA 分别定量细胞摄取 [ 3 H] 胸苷或 BrdUrd。 将HUVECs以5×10³个细胞/孔接种到96孔板中,血清饥饿12小时。加入塞马西尼(SU-5416)(0.05-5μM)预处理1小时后,用VEGF(50ng/mL)刺激细胞。72小时后,采用四唑盐法检测细胞活性并计算IC₅₀值。蛋白质印迹分析中,用0.5-2μM药物和VEGF处理HUVECs,裂解后与抗磷酸化VEGFR2、Akt、ERK1/2和GAPDH的抗体孵育[1] 将VSMCs接种到96孔板中,用塞马西尼(SU-5416)(0.5-10μM)预处理1小时后,用PDGF-BB(20ng/mL)刺激细胞。48小时后,通过BrdU掺入法评估细胞增殖。用1μM药物处理A549细胞24小时,采用酶联免疫吸附试验(ELISA)测量VEGF/bFGF的分泌量[2] 用塞马西尼(SU-5416)(0.1-10μM)单独或与沙利度胺(10μM)联合处理A375和SK-MEL-28黑色素瘤细胞72小时,采用MTT法检测细胞活性以评估协同细胞毒性[5] |
| 动物实验 |
Mice: At 12 weeks of age, female BALB/c nu/nu mice weighing 20–22 g are utilized. In this surgical procedure, aseptic technique is applied. The abdominal wall just above the colon has a tiny 1 cm midline incision made in it. Applying a 27-gauge needle beneath the colon's serosa allows for the implantation of C6 cells (0.5×10 6 cells/animal). All of the exposed intestine is reinserted into the abdominal cavity following implantation. Using a 6.0 surgical suture and wound clips, the peritoneum and skin are sealed. Seven days following surgery, the wound clips are extracted. Starting one day after implantation, the animals receive a 50 μL intraperitoneal bolus injection of either DMSO or Semaxanib (SU5416) once a day. The animals are put to sleep 13–16 days after implantation, and the amount of local tumor growth on the colon is measured using venier calipers or by weighing the tumors. The formula for calculating tumor volumes is length × width × height.
Rats: Five groups of sixty male Sprague Dawley rats (n = 60, 6–8 weeks) are randomly assigned to: control (Con), pneumonectomy (PNx), Semaxanib (SU), semaxinib+hypoxia (SuHx), and semaxinib+PNx (SuPNx). It uses the SuHx protocol. In short, animals receive an injection of 25 mg/kg of semaxinib dissolved in carboxymethylcellulose (CMC) and are then exposed to 10% hypoxia for four weeks before being returned to normoxia. Animals from PNx had a left pneumonectomy. A 25 mg/kg injection of semaxinib is given two days after PNx surgery. Con received only the CMC that was solvent. Echocardiography is used to assess the morphometry and function of the heart at baseline (prehypoxia/presurgery), week 2, and week 6. The animals are put to sleep and their left and right ventricles' (LV and RV) pressures are measured using catheterization two and six weeks after surgery or hypoxia. Pharmacokinetics [5] Semaxanib plasma and urine sampling [5] Blood samples for semaxanib were collected on day 1 of the first and second course of treatment. Blood samples (5 ml) were collected in heparinized tubes predose and at 10, 35, and 45 min, 1, 1.5, 2, 4, 6, 8, and 24 h after the semaxanib infusion. Total urinary volume was collected from 0–8, 8–24, 24–48, and 48–72 h during the first week of course 1. Immediately after collection, all samples were centrifuged at 3,000 rpm for 15 min, transferred to labeled cryostorage tubes, and frozen at −80°C until analysis. Pharmacokinetic analyses for Semaxanib [5] Non-compartmental modeling and parameter estimation were performed using WinNonLin®. The area under the concentration–time curve from time zero to the time of the final quantifiable sample (AUC0–Tf) was calculated using the linear trapezoid method. The AUC was extrapolated to infinity (AUC0–inf) by dividing the last measured concentration by the terminal rate constant (k), which was calculated as the slope of the log-linear terminal portion of the plasma concentration–time curve using linear regression. The terminal phase half-life (t ½) was calculated as 0.693/k. The observed maximum plasma concentration (C max) and the time to maximum concentration (T max) were determined by inspection of the concentration–time curve. Rats were randomly divided among five groups: control (Con), Semaxanib/SU5416 (SU), pneumonectomy (PNx), SU5416 + hypoxia (SuHx), and SU5416 +PNx (SuPNx) (Fig. 1). The SuHx protocol was employed as previously described (5). Briefly, animals were injected with SU5416 (25 mg/kg) dissolved in carboxymethylcellulose (CMC) and exposed to hypoxia (10%) for 4 wk followed by re-exposure to normoxia. PNx animals underwent a left pneumonectomy. Two days following PNx surgery an injection of SU5416 was administered (25 mg/kg). The Con group received only the solvent CMC. Echocardiography was utilized at baseline (prehypoxia/presurgery), week 2, and week 6 to determine cardiac morphometry and function. Two and six weeks postsurgery/posthypoxia animals were anesthetized and right ventricle (RV) and left ventricle (LV) pressure measurements via catheterization were performed. Animals were killed via exsanguination, and organs were weighed and processed for analysis.[3] The study was performed using an established model of PAH in mice that is generated by hypoxia exposure combined with Semaxanib (SU5416) administration (HPX+SU model). Healthy normoxic mice (NMX group) and healthy hypoxic mice (HPX group) were used as controls. The HPX+SU murine PAH model has been well-characterized in previous studies. Briefly, ten-weak-old male C57BL/6 mice (Charles River Laboratories) were exposed to normobaric hypoxia (10% of oxygen) for 3 weeks (n = 25) and were only removed from the chamber once per week for the administration of subcutaneous injections of the VEGF inhibitor, Semaxanib/SU5416. SU5416 was suspended in carboxymethyl cellulose (CMC) (0.5% [w/v] CMC sodium, 0.9% [w/v] sodium chloride, 0.4% [v/v] polysorbate 80, 0.9% [v/v] benzyl alcohol in deionized water) and injected at 20 mg/kg. HPX mice (n = 12) were exposed to the same hypoxic conditions and weekly sham injections. NMX mice (n = 25) were maintained in a room with normal oxygen levels.[4] Nude mice bearing Colo205, MDA-MB-231, or A549 xenografts (100-150 mm³) were randomly divided into control and treatment groups. Semaxanib (SU-5416) was dissolved in DMSO and diluted with saline (final DMSO concentration ≤ 5%), then administered intraperitoneally at 20 mg/kg/day for 21 days. Tumor volume was measured every 3 days, and mice were euthanized to collect tumors for CD31 immunostaining [1] Lewis lung carcinoma cells were implanted into C57BL/6 mice. When tumors reached 50 mm³, mice were treated with Semaxanib (SU-5416) (10 mg/kg/day, i.p.) for 14 days. Intravital multi-fluorescence videomicroscopy was used to observe tumor microcirculation on days 7 and 14 [2] Male Sprague-Dawley rats underwent left pneumonectomy, followed by subcutaneous injection of Semaxanib (SU-5416) at 20 mg/kg/week for 3 weeks. Mean pulmonary arterial pressure was measured by catheterization, and lung tissues were collected for histopathological analysis of vascular remodeling [3] |
| 药代性质 (ADME/PK) |
Pharmacokinetics and pharmacodynamics [5]
All 12 patients had plasma sampling performed for Semaxanib in course 1, and 7 patients in course 2. The principal PK parameters for semaxanib are summarized in Table 5. C max ranged from 1.2 to 3.8 μg/ml in course 1 and 1.1 to 3.9 μg/mL in course 2; t 1/2 was 1.3 ( ± 0.31) h. The PK parameters from the first and second courses were similar, suggesting neither major drug accumulation nor drug interactions. Additionally, PK parameters of exposure were similar to those observed in single-agent phase II studies, further supporting the conclusion of absence of drug interactions. Scatterplots of C max and AUC values of semaxanib for the seven patients having PK sampling on course 1 and course 2 are depicted in Figs. 1 and 2. Comparison of the C max and AUC values between cycle 1 and 2 showed no significant differences over time for C max but a decrease in the AUC values in cycle 2 (not statistically significant). Pharmacokinetic results demonstrate Semaxanib parameters of exposure comparable to those observed in single-agent phase I studies, suggesting the lack of major drug interactions between semaxanib and thalidomide. Comparison of the C max and AUC values between course 1 and 2 showed no significant differences for C max but a decrease in the AUC values in course 2. This is consistent with results from other studies reporting an induction of clearance of 50–60% for semaxanib on the daily or biweekly dosing schedule. The mechanism of the increase in clearance is not known but may be secondary to liver enzyme induction either by the drug or by the corticosteroids required for premedication. The PD analysis revealed a trend toward an increase in serum VEGF levels for the patients receiving more than four cycles of treatment. However, no statistical comparisons could be performed in this small patient population. This observation appears consistent with recent studies suggesting that high urinary and plasma levels of VEGF may correlate with clinical response to a VEGFR inhibitor. One possible explanation for these findings may be the decrease of internalized VEGF following receptor binding during the treatment with a VEGFR inhibitor thus leading to an increase in circulating VEGF levels. Limited data suggest that Semaxanib (SU5416) has poor oral bioavailability and is typically administered intravenously or subcutaneously in preclinical studies. Plasma protein binding is high, but clinical drug-drug interactions due to protein displacement are unlikely. [9] In patients with advanced solid tumors, intravenous administration of Semaxanib (SU-5416) (65 mg/m² twice weekly) showed a mean plasma half-life of 2.8 hours, Cmax of 1.2 μg/mL, and AUC₀-∞ of 3.6 μg·h/mL. The drug was metabolized primarily by cytochrome P450 3A4, with 70% of the dose excreted in feces and 25% in urine within 72 hours [5] In rats, single intravenous injection of Semaxanib (SU-5416) (20 mg/kg) resulted in a volume of distribution of 1.8 L/kg and total body clearance of 0.5 L/h/kg [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Non-hematological toxicities of Semaxanib [5]
The principal non-hematological toxicities of the regimen were headache in eight patients, that reached grade 3–4 in three patients and grade 3–4 thrombosis in three patients. Patients who experienced severe headache were all female, aged 43–59, with a history of migraine (two patients) or anxiety (one patient). This side effect occurred predominantly after the first infusion of semaxanib and decreased to a grade 1 or 2 with premedication using non-steroidal anti-inflammatory drugs for subsequent infusions. One patient, however, decided to withdraw consent after experiencing a grade 3 headache in course 1. One patient experienced a pulmonary embolism on day 20 of the first course. Two additional patients experienced grade 3 thrombosis of the internal jugular vein and subclavian vein, respectively. For both these patients, no extension to the vena cava and no sign of pulmonary embolism was detected on a spiral computerized tomography scan. These thromboembolic events were considered drug related and affected patients were discontinued from study as required by the protocol. Sensory neuropathy, described as intermittent tingling and numbness mainly in the upper and lower extremities was observed in eight patients but generally mild to moderate: grade 1 for five patients and grade 2 for two patients. Only one patient experienced grade 3 sensory neuropathy after the first course. The same patient experienced additional neurological symptoms including headache, vertigo, loss of balance and was diagnosed with brain metastases. Lower extremity edema was a frequent but tolerable side effect (grade 1 for two patients and grade 2 for three patients). The severity of the edema appeared to increase with the number of courses of treatment received. Other toxicities were mild to moderate (grade 1 or 2) and included: asthenia (11 patients), constipation (3 patients), hypercholesterolemia (1 patient), and hyperglycemia (2 patients). One patient experienced asymptomatic grade 4 hypertriglyceridemia after four courses of treatment and received atorvastatin calcium with significant lowering of triglyceride levels permitting continued study participation at a lower dose level. Two patients experienced asymptomatic grade 4 hyperglycemia related to the corticosteroid therapy required as premedication for semaxanib. Of note, no significant changes were observed in serum cortisol levels or coagulation tests performed during treatment; however only six patients completed these laboratory tests according to the protocol. Principal non-hematological toxicities are summarized in Table 4. Hematological toxicity of Semaxanib[5] Hematological toxicity was minimal and included grade 1 anemia in three patients. No neutropenia, lymphopenia, or thrombocytopenia was observed. We showed that the VEGFR-2 tyrosine kinase inhibitor Semaxanib/SU5416 is well tolerated at a dose of 65 mg/m2 twice weekly in combination with weekly cisplatin 30 mg/m2 and irinotecan 50 mg/m2 weeks 1-4 every 6 weeks. Toxicity was overall reversible, manageable, and consistent with the previously reported toxicity profile of SU5416. At DL2, we observed an increase in hematologic toxicity over what is expected for weekly cisplatin and irinotecan, suggesting that SU5416 at higher doses may exacerbate the hematologic toxicity of this regimen. Pharmacokinetic analyses were not performed in our study, which limits our ability to fully explain this finding, and we cannot exclude that pharmacokinetic interactions resulted in increased or overlapping toxicity at DL2. Our study population was heavily pre-treated with more than 3/4 of the patients having received 2 or more lines of prior therapy, which may have limited the patients' ability to tolerate the investigational treatment regimen. Despite administration of low-dose antithrombotic prophylaxis, we observed an increase in treatment-related venous thromboembolic events over what is expected for Semaxanib/SU5416 (0-16%) alone. Both cisplatin and irinotecan are associated with venous thromboembolism, and the use of these agents in combination with SU5416 may have contributed to the incidence of venous thromboembolic events observed on our study. It has been hypothesized that the combination of SU5416 with agents that induce thrombocytopenia may lead to an increase in thromboembolic events due to a combined effect on platelets and endothelium, which may also explain our findings. [6] In clinical trials, Semaxanib (SU5416) was generally well-tolerated, with side effects including nausea, vomiting, and mild hematologic toxicity. No severe organ toxicity was reported in animal studies. [5][6] Rats treated with Semaxanib (SU-5416) (20 mg/kg/week, s.c.) combined with pneumonectomy developed severe pulmonary arterial hypertension, characterized by medial hypertrophy of pulmonary arterioles and right ventricular hypertrophy [3] In phase II clinical trials, common adverse events of Semaxanib (SU-5416) included fatigue (62%), nausea (58%), vomiting (45%), diarrhea (38%), and hypertension (30%). Grade 3/4 toxicities included neutropenia (15%), thrombocytopenia (10%), and reversible liver enzyme elevation (8%) [5] The plasma protein binding rate of Semaxanib (SU-5416) was ~95% in human plasma as determined by equilibrium dialysis [6] |
| 参考文献 |
|
| 其他信息 |
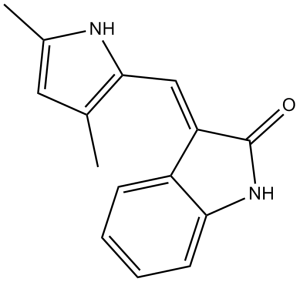
Semaxanib is an oxindole that is 3-methyleneoxindole in which one of the hydrogens of the methylene group is replaced by a 3,5-dimethylpyrrol-2-yl group. It has a role as an antineoplastic agent, a vascular endothelial growth factor receptor antagonist, an EC 2.7.10.1 (receptor protein-tyrosine kinase) inhibitor and an angiogenesis modulating agent. It is a member of pyrroles, a member of oxindoles and an olefinic compound. It is functionally related to a 3-methyleneoxindole.
Semaxanib is a quinolone derivative with potential antineoplastic activity. Semaxanib reversibly inhibits ATP binding to the tyrosine kinase domain of vascular endothelial growth factor receptor 2 (VEGFR2), which may inhibit VEGF-stimulated endothelial cell migration and proliferation and reduce the tumor microvasculature. This agent also inhibits the phosphorylation of the stem cell factor receptor tyrosine kinase c-kit, often expressed in acute myelogenous leukemia cells. Drug Indication Investigated for use/treatment in colorectal cancer and lung cancer. SU5416, a novel synthetic compound, is a potent and selective inhibitor of the Flk-1/KDR receptor tyrosine kinase that is presently under evaluation in Phase I clinical studies for the treatment of human cancers. SU5416 was shown to inhibit vascular endothelial growth factor-dependent mitogenesis of human endothelial cells without inhibiting the growth of a variety of tumor cells in vitro. In contrast, systemic administration of SU5416 at nontoxic doses in mice resulted in inhibition of subcutaneous tumor growth of cells derived from various tissue origins. The antitumor effect of SU5416 was accompanied by the appearance of pale white tumors that were resected from drug-treated animals, supporting the antiangiogenic property of this agent. These findings support that pharmacological inhibition of the enzymatic activity of the vascular endothelial growth factor receptor represents a novel strategy for limiting the growth of a wide variety of tumor types.[1] Vascular endothelial growth factor (VEGF) plays a fundamental role in mediating tumor angiogenesis and tumor growth. Here we investigate the direct effect of a novel small molecule inhibitor of the Flk-1-mediated signal transduction pathway of VEGF, SU5416, on tumor angiogenesis and microhemodynamics of an experimental glioblastoma by using intravital multifluorescence videomicroscopy. SU5416 treatment significantly suppressed tumor growth. In parallel, Semaxanib/SU5416 demonstrated a potent antiangiogenic activity, resulting in a significant reduction of both the total and functional vascular density of the tumor microvasculature, which indicates an impaired vascularization as well as significant perfusion failure in treated tumors. This malperfusion was not compensated for by changes in vessel diameter or recruitment of nonperfused vessels. Analyses of the tumor microcirculation revealed significant microhemodynamic changes after angiogenesis blockage such as a higher red blood cell velocity and blood flow in remnant tumor vessels when compared with controls. Our results demonstrate that the novel antiangiogenic concept of targeting the tyrosine kinase of Flk-1/KDR by means of a small molecule inhibitor represents an efficient strategy to control growth and progression of angiogenesis-dependent tumors. This study provides insight into microvascular consequences of Flk-1/KDR targeting in vivo and may have important implications for the future treatment of angiogenesis-dependent neoplasms.[2] The SU5416/Semaxanib + hypoxia (SuHx) rat model is a commonly used model of severe pulmonary arterial hypertension. While it is known that exposure to hypoxia can be replaced by another type of hit (e.g., ovalbumin sensitization) it is unknown whether abnormal pulmonary blood flow (PBF), which has long been known to invoke pathological changes in the pulmonary vasculature, can replace the hypoxic exposure. Here we studied if a combination of SU5416 administration combined with pneumonectomy (PNx), to induce abnormal PBF in the contralateral lung, is sufficient to induce severe pulmonary arterial hypertension (PAH) in rats. Sprague Dawley rats were subjected to SuPNx protocol (SU5416 + combined with left pneumonectomy) or standard SuHx protocol, and comparisons between models were made at week 2 and 6 postinitiation. Both SuHx and SuPNx models displayed extensive obliterative vascular remodeling leading to an increased right ventricular systolic pressure at week 6 Similar inflammatory response in the lung vasculature of both models was observed alongside increased endothelial cell proliferation and apoptosis. This study describes the SuPNx model, which features severe PAH at 6 wk and could serve as an alternative to the SuHx model. Our study, together with previous studies on experimental models of pulmonary hypertension, shows that the typical histopathological findings of PAH, including obliterative lesions, inflammation, increased cell turnover, and ongoing apoptosis, represent a final common pathway of a disease that can evolve as a consequence of a variety of insults to the lung vasculature. [3] Purpose: This phase II study evaluated the combination of Semaxanib, a small molecule tyrosine kinase inhibitor of vascular endothelial growth factor (VEGF) receptor-2, and thalidomide in patients with metastatic melanoma to assess the efficacy, tolerability, pharmacokinetic (PK) and pharmacodynamic (PD) characteristics of the combination. Patients and methods: Patients with metastatic melanoma, who had failed at least one prior biologic and/or chemotherapeutic regimen, were treated with escalating doses of thalidomide combined with a fixed dose of Semaxanib. Results: Twelve patients were enrolled and received 44 courses of Semaxanib at the fixed dose of 145 mg/m2 intravenously twice-weekly in combination with thalidomide, commencing at 200 mg daily with intrapatient dose escalation as tolerated. Treatment with semaxanib was initiated 1 day before thalidomide in the first course, permitting the assessment of the PKs of semaxanib alone (course 1) and in combination with thalidomide (course 2). The principal toxicities included deep venous thrombosis, headache, and lower extremity edema. Of ten patients evaluable for response, one complete response lasting 20 months and one partial response lasting 12 months were observed. Additionally, four patients had stable disease lasting from 2 to 10 months. The PKs of semaxanib were characterized by drug exposure parameters comparable to those observed in single-agent phase II studies, indicating the absence of major drug-drug interactions. Maximum semaximib plasma concentration values were 1.2-3.8 microg/ml in course 1 and 1.1-3.9 microg/ml in course 2. The mean terminal half-life was 1.3 ( +/- 0.31) h. Biological studies revealed increasing serum VEGF concentrations following treatment in patients remaining on study for more than 4 months. Conclusion: The combination of semaxanib and thalidomide was feasible and demonstrated anti-tumor activity in patients with metastatic melanoma who had failed prior therapy. Further evaluations of therapeutic strategies that target multiple angiogenesis pathways may be warranted in patients with advanced melanoma and other malignancies. [5] Background: This phase I study evaluated the safety of Semaxanib/SU5416, a potent and selective inhibitor of the vascular endothelial growth factor (VEGF) receptor tyrosine kinase Flk-1, in combination with weekly cisplatin and irinotecan in patients with advanced solid tumors. Methods: The patients received cisplatin 30 mg/m² and irinotecan 50 mg/m² weekly from week 1 to week 4, with SU5416 at either 65 mg/m² (dose level (DL)1) or 85 mg/m² (DL2) twice weekly for 6 weeks (1 cycle). Serial ¹⁸fluorodeoxyglucose-positron emission tomography (¹⁸FDG-PET) and ¹⁵O-H₂O-PET scans were obtained. Results: 13 patients were treated (7 on DL1, 6 on DL2); 7 patients completed at least 1 cycle of treatment. 3 patients experienced dose-limiting toxicity (DLT) at DL2 (grade 3 neutropenia and grade 3 thrombocytopenia causing treatment delay, grade 3 nausea/vomiting). No objective responses were observed at DL1, which was determined to be the maximum tolerated dose (MTD). 1 partial response (PR) was observed at DL2. ¹⁸FDG-PET responses were documented but did not predict response according to the Response Evaluation Criteria in Solid Tumors (RECIST). Conclusions: SU5416 at 65 mg/m² twice weekly combined with cisplatin and irinotecan weekly for 4 of 6 weeks is well tolerated but without evidence of clinical activity. ¹⁸FDG-PET may be a useful pharmacodynamic marker of SU5416 bioactivity but requires additional development.[6] Semaxanib (SU-5416) is a small-molecule inhibitor that exerts antitumor effects primarily by blocking VEGF-mediated angiogenesis, as it has minimal direct cytotoxicity against tumor cells [1] The ability of Semaxanib (SU-5416) to induce pulmonary arterial hypertension in animal models highlights its potential cardiovascular toxicity, which requires careful monitoring in clinical use [3] Clinical studies have shown limited single-agent efficacy of Semaxanib (SU-5416) in advanced solid tumors, but it may have synergistic effects when combined with chemotherapy or other targeted agents [5,6] |
| 分子式 |
C15H14N2O
|
|---|---|
| 分子量 |
238.28
|
| 精确质量 |
238.11
|
| 元素分析 |
C, 75.61; H, 5.92; N, 11.76; O, 6.71
|
| CAS号 |
204005-46-9
|
| 相关CAS号 |
(Z)-Semaxanib;194413-58-6; 204005-46-9; 1055412-47-9 (Semaxanib analog/chlorinated)
|
| PubChem CID |
5329098
|
| 外观&性状 |
Yellow to orange solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
481.4±45.0 °C at 760 mmHg
|
| 闪点 |
244.9±28.7 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.684
|
| LogP |
2.87
|
| tPSA |
44.89
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
1
|
| 可旋转键数目(RBC) |
1
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
377
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C1/C(=C(/[H])\C2=C(C([H])([H])[H])C([H])=C(C([H])([H])[H])N2[H])/C2=C([H])C([H])=C([H])C([H])=C2N1[H]
|
| InChi Key |
WUWDLXZGHZSWQZ-WQLSENKSSA-N
|
| InChi Code |
InChI=1S/C15H14N2O/c1-9-7-10(2)16-14(9)8-12-11-5-3-4-6-13(11)17-15(12)18/h3-8,16H,1-2H3,(H,17,18)/b12-8-
|
| 化学名 |
(3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-1H-indol-2-one
|
| 别名 |
Sugen 5416; Sugen5416; Sugen-5416; semoxind; SU5416; SU-5416; SU 5416; Semaxanib; Semaxinib; 204005-46-9; 194413-58-6
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.49 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: 2.25 mg/mL (9.44 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 22.5 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 1% DMSO+30% polyethylene glycol+1% Tween 80: 30 mg/mL 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1967 mL | 20.9837 mL | 41.9674 mL | |
| 5 mM | 0.8393 mL | 4.1967 mL | 8.3935 mL | |
| 10 mM | 0.4197 mL | 2.0984 mL | 4.1967 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00017316 | Completed | Drug: semaxanib Drug: thalidomide |
Melanoma (Skin) |
National Cancer Institute (NCI) |
March 2001 | Phase 2 |
| NCT00006002 | Completed | Drug: semaxanib Drug: dexamethasone |
Prostate Cancer | University of Chicago | June 2000 | Phase 2 |
| NCT00026260 | Completed | Drug: semaxanib | Cervical Cancer | Gynecologic Oncology Group | October 2003 | Phase 2 |
| NCT00005042 | Completed | Drug: semaxanib | Sarcoma | AIDS Malignancy Consortium | November 2000 | Phase 2 |
| NCT00005822 | Completed | Drug: semaxanib Drug: tamoxifen |
Breast Cancer | Case Comprehensive Cancer Center | April 2000 | Phase 1 |
|
|
|
|