| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Human neutrophil elastase
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:西维来司他抑制中性粒细胞向内皮激酶的粘附和迁移 测定:西维来司他(ONO5046;LY544349;EI546)是人中性粒细胞弹性蛋白酶的竞争性抑制剂(IC50 = 44 nM;Ki=200 nM);还抑制从兔、大鼠、仓鼠和小鼠获得的白细胞弹性蛋白酶。细胞测定:HUVEC 在纤连蛋白 (25 μg/mL) 包被的 Falcon 细胞培养插入物上生长至汇合。将 PAF (0.1 mM) 刺激的中性粒细胞添加到 HUVEC 单层(上室)中。将上室暴露于 2 mL HUMEDIA 中,并在不存在或存在 (50 μg/mL) 西维来司他的情况下在 37 ℃ 下再水化 1 小时。随后,移除上室并收集下室中的流体。
|
||
| 体内研究 (In Vivo) |
术中给予西维来司他可有效减少仔猪模型体外循环后肺部中性粒细胞的诱导和活化,改善氧合。西维来司他通过抑制中性粒细胞弹性蛋白酶,直接作用于积聚和活化的白细胞,有效防止氧自由基的产生和细胞因子。
在体内研究中,ONO-5046通过气管内给药抑制了仓鼠的肺出血(ID50=82微克/千克),并通过静脉给药增加了豚鼠的皮肤毛细血管通透性(ID50=9.6毫克/千克)。这两种情况都是由人中性粒细胞弹性蛋白酶诱导的。[3] 当sivelestat(ONO-5046,0.021-2.1 mg/kg)时,人中性粒细胞弹性蛋白酶可抑制仓鼠肺出血(ID50 = 82 pg/kg),并增加豚鼠皮肤毛细血管通透性(ID50 = 9.6 mg/kg)。 kg,气管内)静脉内给药[1]。在大鼠中,通过尾静脉输注西维来司他 (10 mg/kg) 可减少失血性休克后的肺损伤[Int J Mol Med. 2007 Feb;19(2):237-43.]。在大鼠膀胱中,伊来司他(15、60 mg/kg,腹腔注射)可预防缺血再灌注损伤[Mol Cell Biochem. 2008 Apr;311(1-2):87-92.]。 失血性休克后复苏(HSR)引起肺中性粒细胞隔离,导致急性肺损伤(ALI)。中性粒细胞弹性蛋白酶(NE)被认为在ALI的发病机制中起关键作用。本研究探讨特异性NE抑制剂西维司他是否能减轻HSR所致大鼠ALI。雄性Sprague-Dawley大鼠通过抽血使失血性休克维持平均动脉血压30+/-5 mm Hg 60 min,然后用流出的血复苏。hsr治疗的动物在复苏开始时静脉注射西司他(10 mg/kg),随后在复苏阶段或载药中连续注射60分钟(10 mg/kg/h)。通过肺组织学、肺干重比(W/D)、髓过氧化物酶(MPO)活性、肿瘤坏死因子(TNF)- α和诱导型一氧化氮合酶(iNOS)基因表达、核因子(NF)-kappaB DNA结合活性、细胞间粘附分子(ICAM)-1免疫组化分析评估肺损伤。HSR治疗引起肺损伤,表现为肺水肿伴中性粒细胞浸润,肺W/D比、MPO活性、tnf - α和iNOS基因表达、NF-kappaB dna结合活性升高,ICAM-1表达增强。相比之下,西司他治疗显著改善了hsr诱导的肺损伤,从所有这些指标的显着改善来判断。这些结果表明,除了对NE的直接抑制作用外,西司他至少在一定程度上通过抑制炎症信号通路来减轻hsr诱导的肺损伤。[Int J Mol Med. 2007 Feb;19(2):237-43.] 本研究探讨了中性粒细胞弹性酶抑制剂西维司他钠对大鼠膀胱缺血再灌注损伤的影响。用小夹子夹住大鼠腹主动脉,诱导膀胱缺血再灌注损伤。8周龄雄性大鼠分为4组;假手术对照大鼠、30 min缺血-60 min再灌注(IR)大鼠和15、60 mg/kg西司他钠处理的IR大鼠。缺血诱导前60分钟,腹腔注射西司他钠。采用激光多普勒流量计和NO选择电极同时实时监测大鼠血流和一氧化氮(NO)释放。测定大鼠膀胱中NO2-NO3和丙二醛(MDA)的浓度。夹紧腹主动脉,血流量迅速减少,一氧化氮释放量逐渐增加。取下夹子后,血流量迅速增加,一氧化氮释放逐渐恢复到基础水平。这些血流运动和一氧化氮释放被西司他钠水合物以剂量依赖的方式抑制。IR诱导膀胱NO2-NO3和MDA浓度升高,高剂量西司他钠处理膀胱NO2-NO3和MDA浓度显著降低。我们的数据表明,西司他钠可以抑制IR引起的NO2-NO3和MDA浓度的升高,对大鼠膀胱IR损伤具有潜在的保护作用。[Mol Cell Biochem. 2008 Apr;311(1-2):87-92.] |
||
| 酶活实验 |
ONO-5046,N-[2-[4-(2,2-二甲基丙酰氧基)苯磺酰氨基]氨基乙酸,竞争性抑制人中性粒细胞弹性蛋白酶(IC50=0.044微M,Ki=0.2微M)。它还抑制了从兔、大鼠、仓鼠和小鼠中获得的白细胞弹性蛋白酶。然而,ONO-5046即使在100微摩尔下也不会抑制胰蛋白酶、凝血酶、纤溶酶、血浆激肽释放酶、胰腺激肽释放蛋白酶、胰凝乳蛋白酶和组织蛋白酶G[3]。
|
||
| 细胞实验 |
背景:西维来司他钠水合物(Sivelestat)是一种特异性中性粒细胞弹性蛋白酶抑制剂,可有效治疗与全身炎症反应综合征相关的急性肺损伤。因此,它可能有助于治疗肝缺血再灌注损伤(IRI),这是一种中性粒细胞迁移到间质中的情况,导致中性粒细胞弹性蛋白酶从中性粒细胞中释放出来,从而对受影响的组织造成损伤,特别是在肝移植或大面积肝切除后肝功能衰竭的情况下。[2]
目的:本研究旨在探讨西维来司他治疗是否抑制中性粒细胞粘附和向血管壁迁移,并抑制肝脏IRI。[2] 方法:在人脐静脉内皮细胞(HUVEC)模型和大鼠肝IRI模型中检查西维来司他是否以及在多大程度上抑制中性粒细胞的粘附和迁移,并减少肝IRI中的肝损伤。[2] 结果:在HUVEC模型中,发现西维来司他治疗可剂量依赖性地抑制血小板活化因子刺激的中性粒细胞的粘附和迁移程度(p<0.05)。在大鼠模型中,再灌注后12小时,血清肝酶水平显著降低,与对照组相比,治疗组迁移到血管外部位的中性粒细胞数量显著减少(p<0.05)。[2] 结论:西维来司他抑制中性粒细胞在肝IRI中向血管内皮的粘附和迁移,从而抑制肝损伤。[2] |
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Limited information indicates that maternal adalimumab injections produce low levels in breastmilk. Because adalimumab is a large protein molecule, it is likely to be partially destroyed in the infant's gastrointestinal tract and absorption by the infant is probably minimal. Adalimumab was undetectable in the serum of some breastfed infants and some information indicates that adalimumab does not adversely affect the nursing infant. In mothers who received adalimumab during pregnancy, continued use while breastfeeding does not prolong adalimumab elimination by the infant. Most experts and professional guidelines consider adalimumab to be acceptable to use during breastfeeding. Waiting for at least 2 weeks postpartum to resume therapy may minimize transfer to the infant. ◉ Effects in Breastfed Infants One woman with Crohn's disease received adalimumab 40 mg subcutaneously every week during pregnancy and breastfeeding (extent not stated). Her infant demonstrated normal growth and development at 6 months of age. The authors reported a brief follow-up stating that the woman also breastfed her second infant during adalimumab therapy with no adverse consequences. Another woman with Crohn's disease received adalimumab 40 mg subcutaneously every 2 weeks during pregnancy and breastfeeding (extent not stated). Her infant demonstrated normal growth and development at 6 months of age. Two women nursed their infants (extent not stated) while receiving adalimumab 40 mg subcutaneously at unstated intervals for inflammatory bowel disease. They breastfed for at least 21 weeks and 8 weeks, respectively, but the total duration was not stated. At 14.5 and 15 months of age, respectively, neither infant had any signs of adverse drug reactions, allergic reactions or severe infections leading to hospitalization. Developmental milestones were reached on time by both infants. A pregnant woman received adalimumab 40 mg every 2 weeks for Crohn's disease until week 16 of pregnancy. Her infant was exclusively breastfed until 4 months of age and the drug was reinstituted on day 24 postpartum. At 7 months of age, the infant was healthy with normal growth and development. The infant had no infections requiring antibiotics or hospitalization. A case-control study of women with chronic arthritic conditions found 2 women who received adalimumab during pregnancy and lactation (extent not stated). No differences were observed in the 2 infants' growth parameters, developmental milestones, vaccinations and diseases in the first year of life compared to those not exposed to the drugs with lactation. A woman receiving adalimumab for severe psoriasis breastfed 2 infants following 2 pregnancies. No adverse effects were reported in the infant, although the dosage of adalimumab and the extent of breastfeeding were not reported. In a multi-center study of women with inflammatory bowel disease in pregnancy (the PIANO registry), 99 women received adalimumab while breastfeeding their infants. Among those who received adalimumab or another biologic agent while breastfeeding, infant growth, development or infection rate was no different from infants whose mothers received no treatment. An additional 68 women received a biologic agent plus a thiopurine. Infant outcomes were similar in this group. A national prospective registry of patients with rheumatic diseases who were treated with biological DMARDs was conducted in Spain. One whose mother was taking adalimumab was breastfed (extent not stated) with no mild or severe adverse events reported in the infant. A multicenter, retrospective observational study in France reported the outcomes of infants who were breastfed by mothers taking a TNF inhibitor during pregnancy or postpartum for inflammatory bowel disease. Of 153 women who continued anti-TNF therapy postpartum, 55 were taking adalimumab. The exact number of the infants breastfed during maternal adalimumab therapy was not stated. Of the 153 cases, 68 breastfed their infants for a mean duration of 61 days (range 31 to 111 days). Thirty of the breastfed infants were born to mothers who had received an ant-TNF agent after 26 weeks of pregnancy and were likely born with blood levels of the agent. None of the breastfed infants had any infectious complications. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
||
| 参考文献 |
[1]. Interact Cardiovasc Thorac Surg.2008 Oct;7(5):785-.
[2]. Dig Dis Sci.2014 Apr;59(4):787-94. [3]. Biochem Biophys Res Commun. 1991 Jun 14;177(2):814-20. |
||
| 其他信息 |
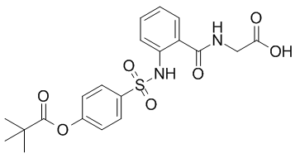
Sivelestat is a N-acylglycine and a pivalate ester. It is functionally related to a N-benzoylglycine.
Sivelestat has been used in trials studying the treatment of Acute Lung Injury and Respiratory Distress Syndrome, Adult. See also: Adalimumab (annotation moved to). ONO-5046, N-[2-[4-(2,2-Dimethylpropionyloxy)phenylsulfonylamino] aminoacetic acid, competitively inhibited human neutrophil elastase (IC50 = 0.044 microM, Ki = 0.2 microM). It also inhibited leukocyte elastase obtained from rabbit, rat, hamster and mouse. However, ONO-5046 did not inhibit trypsin, thrombin, plasmin, plasma kallikrein, pancreas kallikrein, chymotrypsin and cathepsin G even at 100 microM. In in vivo studies, ONO-5046 suppressed lung hemorrhage in hamster (ID50 = 82 micrograms/kg) by intratracheal administration and increase of skin capillary permeability in guinea pig (ID50 = 9.6 mg/kg) by intravenous administration, both of which were induced by human neutrophil elastase.[3] What is known and objective: This article summarizes the effects of sivelestat on acute lung injury/acute respiratory distress syndrome (ALI/ARDS) or ARDS with coagulopathy, both of which are frequently seen in patients with COVID-19. Comment: COVID-19 patients are more susceptible to thromboembolic events, including disseminated intravascular coagulation (DIC). Various studies have emphasized the role of neutrophil elastase (NE) in the development of DIC in patients with ARDS and sepsis. It has been shown that NE inhibition by sivelestat mitigates ALI through amelioration of injuries in alveolar epithelium and vascular endothelium, as well as reversing the neutrophil-mediated increased vascular permeability. What is new and conclusions: Sivelestat, a selective NE inhibitor, has not been evaluated for its possible therapeutic effects against SARS-CoV-2 infection. Based on its promising beneficial effects in underlying complications of COVID-19, sivelestat could be considered as a promising modality for better management of COVID-19-induced ALI/ARDS or coagulopathy. Keywords: COVID-19; acute lung injury/acute respiratory distress syndrome; coagulopathy; neutrophil elastase inhibitor; sivelestat.[J Clin Pharm Ther. 2020 Dec;45(6):1515-1519.] |
| 分子式 |
C20H22N2O7S
|
|---|---|
| 分子量 |
434.46
|
| 精确质量 |
434.114
|
| 元素分析 |
C, 55.29; H, 5.10; N, 6.45; O, 25.78; S, 7.38
|
| CAS号 |
127373-66-4
|
| 相关CAS号 |
Sivelestat sodium;150374-95-1;Sivelestat sodium tetrahydrate;201677-61-4
|
| PubChem CID |
107706
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 折射率 |
1.598
|
| LogP |
2.96
|
| tPSA |
147.25
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
30
|
| 分子复杂度/Complexity |
731
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)(C)C(OC1=CC=C(S(=O)(NC2=CC=CC=C2C(NCC(O)=O)=O)=O)C=C1)=O
|
| InChi Key |
BTGNGJJLZOIYID-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H22N2O7S/c1-20(2,3)19(26)29-13-8-10-14(11-9-13)30(27,28)22-16-7-5-4-6-15(16)18(25)21-12-17(23)24/h4-11,22H,12H2,1-3H3,(H,21,25)(H,23,24)
|
| 化学名 |
N-{2-[({4-[(2,2-Dimethylpropanoyl)oxy]phenyl}sulfonyl)amino]benzoyl}glycine
|
| 别名 |
ONO5046, LY544349, EI546; ONO 5046; ONO5046; ONO-5046; LY544349; LY-544349; LY 544349; EI 546 sodium salt hydrate, Elaspol sodium salt hydrate, LY 544349 sodium salt hydrate, Trade name: Elaspol.Ono-5046; 331731-18-1; 2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetic acid; ONO5046; LY544349; ABT-D2E7;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : 87~100 mg/mL ( 200.24~230.17 mM )
Ethanol : 3.03 ~8 mg/mL(~6.97 mM ) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.75 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.75 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.75 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5% DMSO+ 40% PEG300+ 5% Tween 80+ 50% ddH2O: 3.25mg/ml (7.48mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3017 mL | 11.5085 mL | 23.0171 mL | |
| 5 mM | 0.4603 mL | 2.3017 mL | 4.6034 mL | |
| 10 mM | 0.2302 mL | 1.1509 mL | 2.3017 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。