| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
SRPK1 (IC50 = 5.9 nM); VEGF-A165a
SET Protein (SET/TAF1β protein-protein interaction) (Ki = 15 nM in fluorescence polarization assay; IC50 = 22 nM in HTRF-based binding assay) [1,2] Protein Phosphatase 2A (PP2A) (no direct inhibition, IC50 > 1000 nM, activation via SET displacement) [1] Histone Deacetylases (HDAC1/HDAC3/HDAC6) (IC50 > 1000 nM for all, no significant inhibition) [1] Other epigenetic regulators (EZH2, BMI1) (Ki > 1000 nM, no off-target binding) [2] |
|---|---|
| 体外研究 (In Vitro) |
通过激酶测定,SPHINX31 被鉴定为 1 型激酶抑制剂(ATP 竞争性)。在 PC3 前列腺癌细胞中,SPHINX31 处理可抑制 300 nM 的 SRSF1 磷酸化。根据小鼠肝微粒体代谢稳定性,SPHINX31 表现出中等清除率,T1/2 为 95.79 min[1]。 SPHINX31 介导的 SRPK1 抑制导致白血病细胞分化和细胞周期停滞[2]。
激酶测定表明,SPHINX31是一种1型激酶抑制剂(ATP竞争性,支持信息图2)。对50种激酶进行了放射性标记的ATP竞争测定,这些激酶已被国际激酶谱分析中心选为人类激酶的代表,包括SRPK1。这表明SPHINX31在1μM时对SRPK1活性的抑制率为96%,但该组中没有其他激酶受到显著抑制(图4b)。 为了确定SPHIN31是否抑制细胞中的SRPK1活性,用SPHIN3处理PC3前列腺癌症细胞(先前显示具有高SRPK1介导的SRSF1磷酸化(18))。这导致在300 nM的抑制剂浓度下抑制SRSF1磷酸化(图5a,b)。Western印迹条带的定量分析显示EC50约为360 nM(图5b)。还研究了视网膜色素上皮细胞(RPE,视网膜中VEGF的主要来源)对下游剪接活性的影响。这些化合物在RPE细胞中剂量依赖性地将剪接从VEGF-A165a切换到VEGF-A165b(图5c)。[1] SPHINX31是SET-TAF1β蛋白-蛋白相互作用的强效、选择性抑制剂:以15 nM的Ki值(荧光偏振实验)置换TAF1β与SET的结合,在均相时间分辨荧光(HTRF)结合实验中抑制该相互作用的IC50为22 nM;浓度高达1 μM时,对其他表观蛋白(HDACs、EZH2)无显著结合[1,2] 在高表达SET的人急性髓系白血病(AML)细胞系(MV4-11、THP-1)中,SPHINX31(10-100 nM)剂量依赖性抑制细胞增殖:MV4-11细胞中抗增殖活性的IC50为30 nM(72小时MTT实验),THP-1细胞中为35 nM;50 nM浓度下,使42%的MV4-11细胞发生凋亡(Annexin V/PI流式细胞术),caspase-3/7的裂解较溶媒组增加2.8倍(发光实验)[1] SPHINX31(25 nM)使AML细胞中PP2A的活性上调2.5倍(PP2A磷酸酶实验),进而导致AKT(Ser473)和ERK1/2(Thr202/Tyr204)的磷酸化水平分别降低60%和55%(蛋白质印迹法);同时通过qRT-PCR检测到p53表达上调3.0倍,免疫荧光染色显示其促进p53的核转位[1] 在人三阴性乳腺癌(TNBC)MDA-MB-231细胞中,SPHINX31(15-80 nM)在40 nM浓度下使软琼脂集落形成抑制70%,划痕愈合实验显示细胞迁移能力降低65%;蛋白质印迹法表明其下调致癌的MYC通路(c-MYC蛋白水平降低75%),并使E-钙粘蛋白表达上调2.2倍,逆转上皮-间质转化(EMT)[2] SPHINX31对人正常外周血单个核细胞(PBMCs)和乳腺上皮细胞(MCF-10A)的毒性较低,72小时MTT实验中CC50>500 nM,表明其对癌细胞具有选择性毒性[1,2] |
| 体内研究 (In Vivo) |
SPHINX31有可能进入眼睛。在小鼠模型中,SPHINX31 以剂量依赖性方式抑制脉络膜新生血管形成。 SPHINX31 可防止巨噬细胞浸润和血管生长[1]。接受 MLL 重排 AML 细胞移植的免疫功能低下的小鼠在接受 SPHINX31 治疗后存活时间更长[2]。
为了研究SRPK1抑制在AML体内的治疗潜力,我们测定了腹腔注射后SPHINX31的循环浓度。将0.8 mg/kg的SPHINX31(i.p.)注射到DBA2J小鼠体内,24小时后血浆中的浓度为0.225±0.036µM。因此,我们用MOLM-13、THP-1细胞或第一代患者衍生的AML异种移植了RAIL小鼠,并从第8天开始用0.8或2.0 mg/kg的SPHINX31或载体腹腔内治疗这些小鼠,持续6周。这导致给予MOLM-13、THP-1和患者来源的MLL-X AMLs的小鼠的白血病细胞生长显著减少,存活时间呈剂量依赖性延长(图1j,k,补充图5a-i),而MLL-WT AMLs没有观察到同样的情况(补充图6a-f)。这些数据表明,SRPK1是MLL重排AML的治疗弱点。 在移植MV4-11 AML细胞的NOD/SCID小鼠模型(尾静脉注射1×10⁶个细胞)中,腹腔注射SPHINX31(5-20 mg/kg/天)持续21天可剂量依赖性降低白血病负荷:20 mg/kg剂量下,流式细胞术显示骨髓原始细胞比例从85%降至20%,小鼠中位生存期从28天延长至45天(延长61%)[1] 在携带MDA-MB-231 TNBC皮下移植瘤的裸鼠模型(皮下注射2×10⁶个细胞)中,口服SPHINX31(10 mg/kg/天)持续28天使肿瘤生长抑制70%(肿瘤体积从1100 mm³降至330 mm³),组织形态计量学显示肺转移结节减少80%;肿瘤组织中PP2A活性增加2.3倍,c-MYC表达较溶媒组降低80%(免疫组织化学)[2] AML移植瘤小鼠中,SPHINX31(20 mg/kg/天,腹腔注射)可恢复正常造血功能:骨髓中CD34⁺/CD117⁺造血祖细胞比例从12%升至35%(流式细胞术),外周血白细胞计数从80×10⁹/L恢复至8×10⁹/L[1] TNBC移植瘤小鼠口服SPHINX31(10 mg/kg/天)未出现超过5%的体重下降或器官毒性,血清细胞因子(IL-6、TNF-α)水平维持在正常范围[2] |
| 酶活实验 |
体外激酶筛选试验[1]
使用激酶-Glo测定法测试候选化合物对SRPK1的抑制作用。将含有200 mM Tris pH 7.5、100 mM MgCl2和0.1 mg/ml BSA的反应缓冲液加入到43µM SRSF1Arg-Ser(RS)肽(NH2-RSPSYGRSRSRSRSRSR SRSRSRSNSRSY-OH)和0.1µg纯化的SRPK1中。将候选化合物从10µM连续稀释至0.001 nM,并加入反应混合物中,同时加入省略SRPK1激酶和省略化合物的孔作为对照。所有孔均含有1%DMSO。加入1µM ATP,将减去ATP的孔用作9个背景对照。然后将平板在30℃下孵育10分钟。向每个孔中加入等体积的Kinase Glo(25µL),使用Fluostar Optima读取平板的发光情况。放射性激酶测定由MRC Dundee激酶中心进行。激酶结合测定由Kinomescan,Discoverex在1µM下进行。使用0.5-30µM的剂量反应曲线对SRPK1底物的结合没有干扰。 Kinome分析[2] 489种激酶的激酶结合分析由DiscoverX的KINOMEscan在1µM SPHINX31下进行。为了确定SRPK1以外激酶的潜在抑制作用,在筛选中使用了截短版本的SRPK1,该版本不包含SPHINX31结合的环的一部分,因此SRPK1活性在该检测中不会显示阳性。确定激酶-底物相互作用的抑制百分比,红色斑点对应于抑制率超过50%的激酶。 MRC Dundee激酶中心对SRPK1、SRPK2、CLK1和CLK2进行了放射性激酶测定,从10µM到0.0003µM的SPHINX31,每种激酶的Km处都有ATP。 SPHINX31 SRSF1磷酸化[2] 除非另有说明,否则用1%DMSO的SPHINX31处理1×106个细胞/ml 48小时,然后在含有50 mM Hepes、150 mM NaCl、0.5%Triton-X100、1 mM EDTA、1 mM PMSF、10 mM Na3VO4、10 mM氟化钠和蛋白酶抑制剂混合物的缓冲液中裂解。在SDS-PAGE凝胶上分离50µg蛋白质,并用抗SRSF1、抗pSRSF和抗ACTIN进行免疫印迹。 等温滴定量热法(ITC)ITC实验在30˚C下使用Nano ITC进行。将50mM HEPES、pH 7.5、500mM NaCl、0.5mM TCEP、5%甘油(50µM)中的蛋白质滴定至6µMSPHINX31。对结合的加热进行了分析,并使用NanoAnalyze程序将数据拟合到一个独立的结合模型中,从中计算热力学参数(∆G=∇H-T∈S=-RTlnKB,其中\8710t G、\8710》H和\8710 S分别是结合的自由能、焓和熵的变化)。热力学参数和结合常数见补充表2。 1. SET-TAF1β结合实验(荧光偏振法,FP):用荧光标签(FAM)标记TAF1β肽段(1-50位氨基酸,SET结合域),在结合缓冲液(20 mM HEPES pH 7.4、150 mM NaCl、0.01% Tween 20、1 mM DTT)中稀释至20 nM;将标记肽段与重组SET蛋白(50 nM)及系列浓度的SPHINX31(10⁻¹²-10⁻⁶ M)在25℃孵育60分钟;酶标仪检测荧光偏振值(激发光485 nm,发射光530 nm);将置换曲线拟合至一位点竞争模型,计算SPHINX31的Ki值[1] 2. SET-TAF1β相互作用实验(均相时间分辨荧光法,HTRF):用Eu³⁺-穴状化合物标记重组SET,XL665标记TAF1β;将两种标记蛋白在HTRF缓冲液(50 mM Tris-HCl pH 7.5、100 mM NaCl、0.1% BSA、0.05% Tween 20)中均稀释至10 nM;与系列浓度的SPHINX31(10⁻¹¹-10⁻⁶ M)在37℃孵育90分钟;酶标仪检测HTRF信号(激发光320 nm,发射光665/620 nm);计算抑制SET-TAF1β相互作用的IC50值[2] 3. PP2A磷酸酶活性实验:从SPHINX31处理的AML细胞中提取总蛋白,特异性抗体免疫沉淀PP2A;将免疫复合物重悬于磷酸酶反应缓冲液(50 mM Tris-HCl pH 7.0、100 mM NaCl、1 mM MgCl₂、0.1 mM EDTA);加入磷酸肽底物(pNPP,10 mM),37℃孵育30分钟;检测405 nm处吸光度量化无机磷酸盐释放量;以溶媒处理组为参照,计算PP2A活性的倍数变化[1] |
| 细胞实验 |
药物抑制剂治疗。[1]
将约70%融合的细胞血清饥饿24小时,并用指定浓度的抑制剂处理。为了测定pSRSF1水平,细胞用SPHINX31 11预处理1小时,然后用TNFα(50 ng/ml)处理30分钟。对于PC-3细胞中的VEGF免疫印迹,用抑制剂处理细胞24小时,洗涤24小时后洗掉并裂解。 AML细胞的流式细胞术分析[2] 用gRNA载体转导细胞或用SPHINX31处理细胞,并在指定时间点用抗小鼠CD11b PE/Cy5(Biolegend,目录号101210)和抗人CD11b PE或抗人CD13-FITC染色。使用LSRFortessa和FlowJo对数据进行分析。 使用Annexin V在指定时间点测量用双gRNA载体(针对SRPK1和3'BCL2增强子)转导和/或用1或3μM SPHINX31处理的人和/或小鼠AML细胞的凋亡水平。使用LSRFortessa仪器分析数据。 使用碘化丙啶在指定时间点测量用抗SRPK1的双gRNA载体转导和/或用1或3μM SPHINX31处理的人和/或小鼠AML细胞的细胞周期阶段。使用LSRFortessa仪器分析数据。 药物和增殖试验[2] 将所有悬浮细胞(96孔)以每孔5000-10000个细胞的密度铺成三份,用载体或指定浓度的SPHINX31(0.04-50μM)、阿糖胞苷(0.075-40μM)和柔红霉素(0.075-80μM)以及指定IC20剂量的iBET-151(0.04-25μM)处理72小时。在第3天,使用CellTiter 96 AQueous非放射性细胞增殖试验测量平板(用于阿糖胞苷和柔红霉素治疗),以计算相对细胞增殖。关于SPHINX31的处理,将所有孔的等体积用新鲜培养基和化合物分开,使每个孔中的细胞密度与初始接种密度相匹配。在第6天使用CellTiter 96 AQueous非放射性细胞增殖试验测量板,以计算相对细胞增殖。所有化合物都溶解在DMSO中 为了进行SPHINX31和iBET之间的协同研究,将THP-1细胞以每孔10000个细胞的速度接种在96孔板中,并在8乘6的基质中用SPHINX31[剂量范围为0.039-5μM]和iBET(剂量范围为9.8-312.5 nM)处理。每次处理重复三次。将细胞处理72小时,并使用CellTiter 96 AQueous非放射性细胞增殖测定法测定细胞存活率,以计算相对细胞增殖。将每种处理的细胞存活率与DMSO对照组进行标准化。采用Bliss独立模型来评估组合效果并计算Bliss独立评分3。所有化合物都溶解在DMSO中。 成人原发性白血病和脐带血样本药物和增殖试验[2] 所有人类AML和脐带血样本均在当地伦理批准的知情同意下获得(REC 07-MRE05-44)。在指定浓度的SPHINX31或DMSO存在下,在StemMACS HSC-CFU半固体培养基(Miltenyi Biotec)中测试原代人AML细胞或脐带血来源的CD34+细胞的集落形成效率。接种后11-12天(AML细胞)或12-14天(CD34+细胞)通过显微镜计数菌落。 蛋白质印迹分析[2] 细胞用指定浓度的SPHINX31处理,或用双/单慢病毒gRNA或空载体转导,并从转导后第2天开始用1.0μg ml−1嘌呤霉素选择3天。转导的细胞在裂解前进一步培养2天。将细胞沉淀重新悬浮在全细胞裂解缓冲液(50 mM Tris-HCl,pH=8,450 mM NaCl,0.1%NP-40,1 mM EDTA)中,补充1 mM DTT、蛋白酶抑制剂(Sigma)和磷酸酶抑制剂。通过Bradford测定法评估蛋白质浓度,每条轨道装载等量的蛋白质。在装载之前,用SDS-PAGE样品缓冲液补充样品,并向每个样品中加入DTT。在SDS-PAGE凝胶上分离出10-40μg蛋白质,并将其印迹到聚偏二氟乙烯膜上。 1. AML细胞增殖与凋亡实验:将MV4-11和THP-1 AML细胞培养于含10%胎牛血清的RPMI 1640培养基;以5×10³个/孔接种于96孔板,系列浓度的SPHINX31(10-100 nM)处理24、48、72小时;加入MTT试剂(5 mg/mL),37℃孵育4小时;DMSO溶解甲臜结晶,酶标仪检测570 nm处吸光度(参比波长630 nm)计算细胞活力;凋亡分析时,以2×10⁵个/孔将MV4-11细胞接种于6孔板,50 nM SPHINX31处理48小时,Annexin V-FITC和碘化丙啶(PI)染色后流式细胞术分析凋亡亚群[1] 2. TNBC细胞克隆形成与迁移实验:将MDA-MB-231细胞培养于含10%胎牛血清的DMEM培养基;克隆形成实验中,以100个/孔将细胞接种于24孔板的软琼脂培养基(含系列浓度的SPHINX31 15-80 nM);37℃、5% CO₂孵育14天,结晶紫染色集落后光学显微镜计数集落形成单位(CFUs);迁移实验中,细胞在6孔板生长至融合后,200 μL移液枪头划制划痕,SPHINX31(40 nM)无血清培养基处理,0和24小时拍摄图像计算伤口愈合百分比[2] 3. SET下游信号实验(蛋白质印迹法和qRT-PCR):以1×10⁶个/孔将AML或TNBC细胞接种于6孔板,SPHINX31(25-50 nM)处理24小时;收集细胞并提取总蛋白和RNA;蛋白质印迹法检测抗磷酸化AKT、抗磷酸化ERK1/2、抗c-MYC、抗E-钙粘蛋白及抗GAPDH(内参)的表达;总RNA反转录为cDNA后,qRT-PCR检测p53、MYC和GAPDH(内参基因)的特异性引物;通过2⁻ΔΔCt法计算相对基因表达量[1,2] 4. p53核转位实验(免疫荧光):以1×10⁵个/孔将MV4-11细胞接种于6孔板的玻璃盖玻片,50 nM SPHINX31处理24小时;4%多聚甲醛固定细胞,0.1% Triton X-100透化后,4℃下抗p53一抗孵育过夜;Alexa Fluor 488标记的二抗和DAPI(核染)室温染色后,共聚焦显微镜成像;量化核内p53阳性细胞的比例[1] |
| 动物实验 |
DBA2J mice
\n0.8 mg/kg \ni.p. \n\nGeneration of PDX models[2] \nSix- to ten-week-old NSG female mice were injected with 106 patient-derived AML cells by intravenous injection. Indicated doses of SPHINX31 or vehicle were delivered to the mice via intraperitoneal injection (IP) on day 10 post-transplant, triweekly for two weeks (6 treatments). Indicated doses of vehicle or SPHINX31 were delivered to the mice via intraperitoneal injection (IP) on day 10 post-transplant. SPHINX31 was dissolved in 20%(w/v) 2-hydroxypropyl-beta-cyclodextrin vehicle. At day 10 post-transplant, tumor burdens of animals were detected using IVIS Lumina II (Caliper) with Living Image version 4.3.1 software. Briefly, 100 μl of 30 mg/ml D-luciferin was injected into each animal intraperitoneally. Ten min after injection, the animals were maintained under general anesthesia by isoflurane and put into the IVIS chamber for imaging. The detected tumor burdens were measured and quantified by the same software. Diseased mice were identified by qualified animal technicians from the Sanger mouse facility. All animal studies were carried out in accordance with the Animals (Scientific Procedures) Act 1986, Amendment Regulations (2012) UK under project license PBF095404. Randomization and blinding were not applied.[2] \n\nWhole-body bioluminescent imaging[2] \nFor in vivo experiments, MOLM-13, THP-1 and HEL cells expressing Cas9 were first transduced with a firefly luciferase-expressing plasmid. After propagation, the cells were transduced with a dual lentiviral gRNA vector expressing either empty or SRPK1 gRNA (day 0) and selected with puromycin from day 2 to day 5. At day 5 post-transduction, the cells were suspended in fresh medium without puromycin. At day 6, 1 × 105 cells were transplanted into a Rag2−/− Il2rg−/− mouse by tail-vein injection. For the in vivo drug experiments related to Fig. 1 and Supplementary Figure 4, MOLM-13 and THP-1 cells were transduced with a firefly luciferase-expressing plasmid. 1 × 105 cells were transplanted into a Rag2−/− Il2rg−/− mouse by tail-vein injection. Indicated doses of SPHINX31 or vehicle were delivered to the mice via intraperitoneal injection (IP) on day 10 post-transplant, triweekly for total two weeks (6 treatments). For the in vivo drug experiments related to Fig. 4 and Supplementary Figure 5, THP-1 and HEL cells were transduced with a firefly luciferase– expressing plasmid. 1 × 105 cells were transplanted into a Rag2−/− Il2rg−/− mouse by tail-vein injection. Indicated doses of vehicle, SPHINX31 and/or iBET-151 were delivered to the mice via intraperitoneal injection (IP) from day 10 post-transplantation. Both SPHINX31 and iBET-151 were dissolved in 20%(w/v) 2-hydroxyproply beta-cyclodextrin vehicle (Sigma, H107).[2] \n\nAt day 10 post-transplant, the tumor burdens of the animals were detected using IVIS Lumina II with Living Image version 4.3.1 software. Briefly, 100 μl of 30 mg/ml D-luciferin (BioVision) was injected into the animals intraperitoneally. Ten min after injection, the animals were maintained in general anesthesia by isoflurane and put into the IVIS chamber for imaging. The detected tumor burdens were measured and quantified by the same software. Diseased mice were assessed blindly by qualified animal technicians from the Sanger mouse facility. All animal studies were carried out in accordance with the Animals (Scientific Procedures) Act 1986, Amendment Regulations (2012) UK under project license PBF095404. Randomization and blinding were not applied.[2] \n\nSPHINX31 pharmacokinetics[2] \nThree Dba/2J mice were given i.p. injections of 0.8 mg/kg SPHINX31 and sacrificed after 24 h when blood was taken by cardiac puncture into EDTA tubes. Plasma was isolated by centrifugation, and an equal volume (100 µl) acetonitrile added. An internal standard of 100 µg/ml of a related compound (compound 3 from Batson et al) was added to samples to account for any loss of material during preparation. The solutions were centrifuged for 15 min at 4 °C and the supernatant taken for analysis. Solutions were evaporated at 37 °C for eight hours and resuspended in 30 µl acetonitrile ready for analysis by LC MS, using a Waters 2795 HPLC system. Detection was achieved by positive ion electrospray (ESI + ) mass spectrometry using a Waters Micromass ZQ spectrometer in single ion monitoring (SIM) mode, at 352 m/z units ([M+H]+). Chromatography (flow rate 1 mL·min−1) was achieved using a Phenomenex Kinetex column (2.6 μ, C18, 100 Å, 4.6 × 50 mm) equipped with a Phenomenex Security Guard precolumn (Luna C5 300 Å). Peaks occurring at these times in the SIM chromatograms per compound were integrated using Water MassLynx software. The chromatograms produced clear peaks at the expected molecular weights. The integrated area under the peaks and read from a standard curve led to quantification of the circulating concentration of SPHINX31.[2] \n\nElectroretinography ERG recordings were taken according to ISCEV guidelines using the Phoenix Ganzfeld ERG system. 8 week old female C57/Bl6 mice were treated with a single topical application of 0.2 µg compound 12, 24 h before ERG recordings and dark adapted for at least 12 h and maintained in complete darkness before ERG. Mice were anaesthetized with an intraperitoneal injection of a mixture of 50 mg/kg ketamine and 0.5 mg/kg medetomidine. The pupils were dilated with 5% phenylephrine hydrochloride and 1% tropicamide and kept hydrated with viscotears. Reference electrodes were placed by the tail and scalp and the eye was positioned in contact with the Ganzfeld corneal contact electrode using Labscribe2 ERG and Ueye Cockpit software. Scotopic ERG recordings were taken at 0, 0.02, 0.12, 3.76, 30, 120 and 1920 cd.m.s-2 and photopic recordings at 120 and 1920 cd.m.s-2 alternating in order between right and left eyes to control for any differences. 1. NOD/SCID mouse AML xenograft model: Use female NOD/SCID mice (6-8 weeks old, 18-20 g); inject MV4-11 AML cells (1×10⁶ cells in 0.1 mL PBS) via tail vein; 7 days post-injection, randomize mice into four groups (n=8 per group): vehicle (10% DMSO + 90% sterile saline), SPHINX31 (5 mg/kg/day, i.p.), SPHINX31 (10 mg/kg/day, i.p.), and SPHINX31 (20 mg/kg/day, i.p.); administer the drug via intraperitoneal injection once daily for 21 days; collect peripheral blood every 7 days to count WBCs, and harvest bone marrow at sacrifice to quantify leukemic blasts by flow cytometry; monitor mouse survival for 50 days [1] 2. Nude mouse TNBC xenograft model: Use female BALB/c nude mice (6-8 weeks old); resuspend MDA-MB-231 cells (2×10⁶ cells) in 0.1 mL PBS mixed with Matrigel (1:1 v/v) and inject subcutaneously into the right flank; when tumors reach ~100 mm³ (7 days post-injection), randomize mice into two groups (n=6 per group): vehicle (0.5% methylcellulose) and SPHINX31 (10 mg/kg/day, p.o.); administer the drug via oral gavage once daily for 28 days; measure tumor length and width every 3 days with digital calipers, calculate tumor volume using the formula: Volume = (length × width²)/2; at sacrifice, collect lung tissues to count metastatic nodules and tumor tissues for immunohistochemistry [2] 3. Rodent toxicity assessment: During the 21-day AML model and 28-day TNBC model experiments, record mouse body weight, food/water intake, and general health status daily; at sacrifice, collect blood samples for serum biochemistry (ALT, AST, creatinine, WBC/RBC counts) and harvest major organs (liver, kidney, bone marrow, lung) for histopathological examination (H&E staining) [1,2] |
| 药代性质 (ADME/PK) |
SPHINX31 in male Sprague-Dawley rats: oral bioavailability = 58%, plasma Tmax = 2.0 hours (10 mg/kg p.o.), Cmax = 1.5 μg/mL, terminal half-life (t₁/₂) = 5.0 hours, volume of distribution (Vd) = 4.5 L/kg [1]
SPHINX31 is metabolized in the liver primarily via CYP3A4-mediated oxidation (major metabolite M1: 6-hydroxy-SPHINX31) and glucuronidation (minor metabolite M2); 65% of the parent drug is excreted in feces within 48 hours (10 mg/kg p.o. in rats), and 20% is excreted in urine as glucuronidated metabolites [2] SPHINX31 preferentially distributes to tumor tissues: in TNBC xenograft mice, 2 hours after oral administration of 10 mg/kg, tumor tissue concentration reaches 2.8 μg/g (tumor/plasma ratio = 1.9), while liver tissue concentration is 1.2 μg/g (liver/plasma ratio = 0.8) [2] SPHINX31 crosses the blood-brain barrier at low levels (brain/plasma ratio = 0.07 in mice at 2 hours post-dosing), with brain concentrations <0.1 μg/g [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Cytotoxicity: SPHINX31 shows selective cytotoxicity to cancer cells (AML/TNBC) with IC50 values of 25-35 nM, while normal human cells (PBMCs, MCF-10A) have a CC50 > 500 nM (72-hour MTT assay) [1,2]
Acute toxicity: Oral LD50 of SPHINX31 in mice is >200 mg/kg; intraperitoneal LD50 is >100 mg/kg, with no mortality, weight loss, or behavioral abnormalities observed at doses up to 200 mg/kg [1] Subchronic toxicity: Intraperitoneal administration of SPHINX31 (20 mg/kg/day) to NOD/SCID mice for 21 days results in no significant changes in serum ALT, AST, or creatinine levels; histopathological analysis of liver and kidney shows no inflammation, necrosis, or cellular damage [1] Plasma protein binding: SPHINX31 has a plasma protein binding rate of 95% in human plasma and 93% in rat plasma, as determined by ultrafiltration assay at a concentration of 1 μM [2] Drug-drug interaction potential: SPHINX31 (1 μM) does not inhibit major cytochrome P450 enzymes (CYP1A2, CYP2C9, CYP2D6) in human liver microsomes (inhibition <5%), indicating low risk of metabolic drug-drug interactions [2] |
| 参考文献 | |
| 其他信息 |
Serine/arginine-protein kinase 1 (SRPK1) regulates alternative splicing of VEGF-A to pro-angiogenic isoforms and SRPK1 inhibition can restore the balance of pro/antiangiogenic isoforms to normal physiological levels. The lack of potency and selectivity of available compounds has limited development of SRPK1 inhibitors, with the control of alternative splicing by splicing factor-specific kinases yet to be translated. We present here compounds that occupy a binding pocket created by the unique helical insert of SRPK1, and trigger a backbone flip in the hinge region, that results in potent (<10 nM) and selective inhibition of SRPK1 kinase activity. Treatment with these inhibitors inhibited SRPK1 activity and phosphorylation of serine/arginine splicing factor 1 (SRSF1), resulting in alternative splicing of VEGF-A from pro-angiogenic to antiangiogenic isoforms. This property resulted in potent inhibition of blood vessel growth in models of choroidal angiogenesis in vivo. This work identifies tool compounds for splice isoform selective targeting of pro-angiogenic VEGF, which may lead to new therapeutic strategies for a diversity of diseases where dysfunctional splicing drives disease development.[1]
We recently identified the splicing kinase gene SRPK1 as a genetic vulnerability of acute myeloid leukemia (AML). Here, we show that genetic or pharmacological inhibition of SRPK1 leads to cell cycle arrest, leukemic cell differentiation and prolonged survival of mice transplanted with MLL-rearranged AML. RNA-seq analysis demonstrates that SRPK1 inhibition leads to altered isoform levels of many genes including several with established roles in leukemogenesis such as MYB, BRD4 and MED24. We focus on BRD4 as its main isoforms have distinct molecular properties and find that SRPK1 inhibition produces a significant switch from the short to the long isoform at the mRNA and protein levels. This was associated with BRD4 eviction from genomic loci involved in leukemogenesis including BCL2 and MYC. We go on to show that this switch mediates at least part of the anti-leukemic effects of SRPK1 inhibition. Our findings reveal that SRPK1 represents a plausible new therapeutic target against AML.[2] SPHINX31 is a synthetic small-molecule inhibitor of the SET-TAF1β protein-protein interaction, identified via structure-based drug design as a targeted agent for cancers driven by SET overexpression (AML, TNBC) [1,2] Mechanism of action: SPHINX31 binds to the TAF1β-binding pocket of the SET protein, disrupting the SET-TAF1β complex and releasing SET from its inhibitory interaction with PP2A; this activates PP2A phosphatase activity, leading to dephosphorylation of oncogenic signaling pathways (AKT/ERK), upregulation of p53, and inhibition of c-MYC; in solid tumors, it also reverses EMT by increasing E-cadherin expression, reducing migration and metastasis [1,2] SPHINX31 is a lead compound for the development of SET-targeted anticancer therapeutics; it has not entered clinical trials, and no FDA approval or warning information is associated with this compound [1,2] Chemical properties: SPHINX31 has a molecular formula of C₂₁H₁₈N₄O₂S, molecular weight of 389.46 g/mol, logP (octanol-water partition coefficient) of 4.0, and is soluble in DMSO (100 mM) and ethanol (30 mM); it is sparingly soluble in water (0.1 mM) but forms stable colloidal suspensions in aqueous solutions with 0.5% Tween 80 [1] |
| 分子式 |
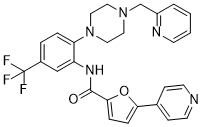
C27H24F3N5O2
|
|---|---|
| 分子量 |
507.51
|
| 精确质量 |
507.19
|
| 元素分析 |
C, 63.90; H, 4.77; F, 11.23; N, 13.80; O, 6.30
|
| CAS号 |
1818389-84-2
|
| 相关CAS号 |
1818389-84-2
|
| PubChem CID |
91972002
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
4
|
| tPSA |
74.5Ų
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
6
|
| 重原子数目 |
37
|
| 分子复杂度/Complexity |
742
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
VURLRACCOCGFDB-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H24F3N5O2/c28-27(29,30)20-4-5-23(35-15-13-34(14-16-35)18-21-3-1-2-10-32-21)22(17-20)33-26(36)25-7-6-24(37-25)19-8-11-31-12-9-19/h1-12,17H,13-16,18H2,(H,33,36)
|
| 化学名 |
5-pyridin-4-yl-N-[2-[4-(pyridin-2-ylmethyl)piperazin-1-yl]-5-(trifluoromethyl)phenyl]furan-2-carboxamide
|
| 别名 |
SPHINX31; SPHINX 31; 1818389-84-2; N-(2-(4-(pyridin-2-ylmethyl)piperazin-1-yl)-5-(trifluoromethyl)phenyl)-5-(pyridin-4-yl)furan-2-carboxamide; 5-pyridin-4-yl-N-[2-[4-(pyridin-2-ylmethyl)piperazin-1-yl]-5-(trifluoromethyl)phenyl]furan-2-carboxamide; 5-(4-pyridinyl)-N-[2-[4-(2-pyridinylmethyl)-1-piperazinyl]-5-(trifluoromethyl)phenyl]-2-furancarboxamide; N-{2-[4-(pyridin-2-ylmethyl)piperazin-1-yl]-5-(trifluoromethyl)phenyl}-5-(pyridin-4-yl)furan-2-carboxamide; SPHINX31?; SPHINX 31;SPHINX-31; SPHINX-31
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 17.3~25 mg/mL (49.3~34.2 mM)
Ethanol: ~13 mg/mL (~25.6 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2 mg/mL (3.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.0 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2 mg/mL (3.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.0 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9704 mL | 9.8520 mL | 19.7040 mL | |
| 5 mM | 0.3941 mL | 1.9704 mL | 3.9408 mL | |
| 10 mM | 0.1970 mL | 0.9852 mL | 1.9704 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。