| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg | |||
| 25mg |
| 靶点 |
orexin receptor/OX
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Suvorexant(也称为 MK-4305)是一种有效的双重 OX 受体拮抗剂,对于 OX1 受体和 OX2 受体的 Ki 值分别为 0.55 nM 和 0.35 nM。 Suvorexant是由默克公司开发的用于治疗失眠的药物。目前正在进行 III 期试验。 Suvorexant 的作用是关闭清醒状态,而不是诱导睡眠。作为一种双重食欲素受体 (OXR) 拮抗剂 (DORA),suvorexant (MK-4305) 已显示出治疗失眠症和睡眠障碍的前景。激酶测定:MK-4305 是 OX1 受体和 OX2 受体的有效拮抗剂,Ki 值分别为 0.55 nM 和 0.35 nM 细胞测定:体外研究表明 MK-4305 具有清晰的辅助特征(对 OX1 受体和 OX2 受体的选择性 >10000 倍) OX2R)通过 MDS Pharma 的 170 种酶、受体和离子通道脱靶筛选确定。
|
||
| 体内研究 (In Vivo) |
在一项小鼠体内研究中,在小鼠非活动阶段(开灯)测试了 suvorexant(25 毫克/千克),此时睡眠自然更普遍且食欲素水平通常较低。研究发现,suvorexant 在给药后的前 4 小时内选择性地增加 REM,从而显着扰乱了睡眠结构。在测试剂量下,suvorexant 仅在第一个小时内显着减少苏醒时间,而 IPSU 不影响苏醒时间。这些数据表明,与 DORA 相比,OX2R 偏好拮抗剂可能降低了扰乱 NREM/REM 结构的趋势
|
||
| 酶活实验 |
MK-4305 对 OX1 受体的 Ki 值为 0.55 nM,对 OX2 受体的 Ki 值为 0.35 nM,使其成为这两种受体的强拮抗剂。
Bioactivation测定[1] 人肝微粒体 在100 mM磷酸钾缓冲液(pH 7.4)中以1 mg/mL蛋白与10 μM测试化合物、1 mM MgCl2、1 mM EDTA、5 mM谷胱甘肽和1 mM NADPH在37℃下预孵育60分钟。用含0.15 μM拉贝他洛尔(内标)的25%乙腈终止反应。将样品涡旋混合,14000 rpm离心10分钟。每个样品的上清液转移到HPLC瓶中进行HRMS分析。 采用uplc -高分辨质谱法(HRMS)对gsh衍生加合物进行鉴定。该系统由一个Waters Acquity样品管理器和两个Waters Acquity UPLC泵组成。HRMS采用Waters Q-TOF Xevo质谱仪进行。采用Phenomenex Synergi 2.5 μm MAX-RP 100 Å柱(50 mm × 2 mm)加热至60°C实现分离。流动相为含有0.1%甲酸的水(溶剂A)和含有0.1%甲酸的乙腈(溶剂B),流速为0.5 mL/min。梯度从第一分钟的5%溶剂B开始,然后在接下来的0.5分钟内线性增加到15%溶剂B。然后在接下来的11.5分钟内将溶剂B增加到50%,然后在2分钟内进一步增加到90%。然后用95%的溶剂B洗涤1.5分钟。在每次运行结束时,在初始条件下重新平衡5分钟。质谱分析采用正离子模式电喷雾电离。ESI毛细管电压为1.5 kV,源温度为100℃,脱溶温度为600℃。质量扫描范围为150 ~ 1000 μ m,扫描时间为0.25 s/次。锁质量为588.8691 amu,使用频率为每5次扫描一次。每个测试化合物形成的谷胱甘肽加合物的相对量是用峰面积比估计的。与gsh衍生加合物相关的质谱峰面积除以内标拉贝他洛尔的面积。 放射配体结合试验[1] 根据Kunapuli等人的方法,从CHO细胞中表达的人食欲素2受体(hOX2R)和食欲素1受体(hOX1R)的Ile408-Val变体制备膜。将CHO/OX2R和CHO/OX1R细胞与PBS/1 mM EDTA分离,1000g离心10分钟。将细胞颗粒在冰冷的20 mM Hepes, 1 mM EDTA, pH 7.4中用Polytron均质,在4℃下20000g离心20分钟。这个过程重复了两次。以5 mg膜蛋白/mL重悬于实验缓冲液(20 mM Hepes, 125 mM NaCl, 5 mM KCl, pH 7.4)中。加入牛血清白蛋白至终浓度为1%,等分液保存于- 80°C。利用自动化Tecan液体处理系统和moser等人描述的Packard unfilter -96进行放射性配体结合试验。实验在96孔微滴板上进行,室温下,最终测定量为1.0 mL,在含125 nM NaCl和5 mM KCl的20 mM Hepes缓冲液(pH 7.4)中进行。用DMSO配制待测化合物溶液,用DMSO连续稀释,10种溶液浓度相差3倍,各20 μL。非特异性结合(NSB)采用高亲和力配体(终浓度为1 μM)测定,总结合(TB)采用DMSO(终浓度为2%)测定。将受体溶液(30pm终值,通常为2−10 μg膜)和氚化配体(~ 80 Ci/ mol)添加到测试化合物中。OX2R受体采用0.15 nM的化合物18 (KD = 0.3 nM)。OX1R受体采用0.7 nM的化合物19 (KD = 3 nM)。使用化合物20在0.03 nM (KD = 0.03 nM)浓度下进行OX1R测定,结果相同;然而,在这种情况下,首先在化合物中加入920 μL的膜,然后再加入60 μL的热配体。室温孵育3小时(化合物20 20小时)后,样品通过Packard GF/B过滤器过滤(预先浸泡在0.2% PEI,聚乙烯西格玛P-3143中),并用1ml 20 mM Hepes冷缓冲液(pH 7.4)洗涤5次。滤板真空干燥后,加入50 μL Packard Microscint-20,用Packard TopCount测定结合放射性(CPM bound)。 放射性配体结合[2] 瞬时表达人OX2受体的HEK293细胞的细胞膜与[3H]-EMPA在Krebs实验缓冲液(8.5 mM HEPES, 1.3 mM CaCl2, 1.2 mM MgSO4, 118 mM NaCl, 4.7 mM KCl, 4 mM NaHCO3, 1.2 mM KH2PO4, 11 mM葡萄糖,pH 7.4)中孵育,总实验体积为0.25 mL,最终DMSO浓度为1%。室温孵育90分钟后,通过GF/B 96孔玻璃纤维板快速过滤,用Tomtec细胞收集机用5 × 0.25 mL的ddH2O洗涤,终止反应。结合放射性是用Lablogic SafeScint通过液体闪烁来测定的,并在微-液体闪烁计数器上检测。非特异性结合被确定为在拮抗剂EMPA达到10 μM饱和浓度的情况下仍然存在。在[3H]-EMPA (0.4 nM - 15 nM)浓度范围内,膜(2 μg蛋白/孔)孵育,进行饱和度研究。使用SafeScint和Beckman LS 6000液体闪烁计数器测定放射性配体浓度。用1.5 nM浓度的[3H]-EMPA和一系列浓度的测试化合物如3 (Suvorexant / MK-4305)孵育膜(2 μg蛋白/孔)进行竞争结合。 通过将相同的细胞膜(2 μg蛋白/孔)添加到含有1% DMSO和1.5 nM辐射配体的Krebs缓冲液的孔中,在不同的时间点上共3小时,测定了辐射配体的结合动力学。通过预平衡膜和[3H]-EMPA测定90 min的解离动力学;然后在不同的时间点加入饱和浓度的冷EMPA (100 μM),以防止放射性配体与受体分离时重新结合。 |
||
| 细胞实验 |
基于 MDS Pharma 对 170 种酶、受体和离子通道的脱靶筛选,体外研究表明 MK-4305 具有清晰的辅助特征(对 OX2R 的选择性>10000 倍)。
FLIPR测定[1] 为了测量细胞内钙,将表达食欲素1受体Ile408-Val变体或人食欲素2受体的中国仓鼠卵巢(CHO)细胞生长在Iscove修饰的DMEM中,该DMEM含有2 mM l-谷氨酰胺、0.5 g/mL G418、1%次黄嘌呤胸腺嘧啶补充剂、100 U/mL青霉素、100 ug/mL链霉素和10%热灭活胎牛血清。将细胞以20000个/孔的速度接种到涂有聚d-赖氨酸的Becton-Dickinson黑色384孔透明底无菌板中。所有试剂均来自GIBCO-Invitrogen Corp.。种板在37°C和6% CO2下孵育过夜。α -6,12人食欲素- a作为激动剂,在1%牛血清白蛋白(BSA)中配制0.5 mM原液,在实验缓冲液(含20 mM HEPES和2.5 mM probenecid, pH 7.4)中稀释,最终浓度为0.3 - 2 nM,用于实验。在DMSO中配制10 mM的原液,然后在384孔板中稀释和移液,首先在DMSO中,然后在分析缓冲液中。实验当天,用100 μL实验缓冲液洗涤细胞3次,然后在60 μL含有1 μM Fluo-4AM酯、0.02% pluronic酸和1% BSA的实验缓冲液中(37°C, 6% CO2)孵育60分钟。然后抽吸染料上样液,用100 μL缓冲液洗涤细胞3次。然后在每个孔中留下30 μL相同的缓冲液。在荧光成像板读取器内,以15 μL的体积向板中加入待测化合物,孵育5 min,最后加入激动剂15 μL。每孔以1 s间隔1 min和6 s间隔4 min测量荧光,并将每个荧光峰的高度与0.3−2 nM ala -6,12 orexin-A用缓冲液代替拮抗剂诱导的荧光峰高度进行比较。对于每种拮抗剂,确定IC50值(抑制50%激动剂反应所需的化合物浓度)。[1] 功能性肌醇磷酸和ERK1/2磷酸化测定[2] 以25 000个细胞/孔密度稳定表达人食欲素-2受体的CHO细胞播种24 h后,在96孔板上进行基于细胞的肌醇磷酸和ERK1/2磷酸化功能测定;完整的分析细节见辅助信息。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Peak concentrations occur at a median Tmax of 2 hours under fasted conditions. Ingestion of suvorexant with a high-fat meal has no effect on AUC or Cmax, but may delay Tmax by approximately 1.5 hours. Mean absolute bioavailability of 10 mg is 82%. Approximately 66% is eliminated in feces and 23% is eliminated in urine. Mean volume of distribution is approximately 49 litres. Metabolism / Metabolites Suvorexant is primarily metabolized by cytochrome-P450 3A4 enzyme (CYP3A4) with a minor contribution from CYP2C19. Major circulating metabolites are suvorexant and a hydroxy-suvorexant metabolite, which is not expected to be pharmacologically active. There is potential for drug-drug interactions with drugs that inhibit or induce CYP3A4 activity. Biological Half-Life Mean half life is approximately 12 hours. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In several clinical trials, suvorexant was found to be well tolerated, with serum ALT elevations in 0 to 5% of patients, usually with higher doses, and resolving spontaneously without dose modification. In the registration trials of suvorexant, there were no reports of clinically apparent liver injury. Suvorexant has been available for a limited period of time, but has yet to be implicated in causing clinically apparent liver injury even with an overdose. Likelihood score: E (unlikely cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Data from two women indicate that amounts of suvorexant in milk are very low. If suvorexant is required by the mother, it is not a reason to discontinue breastfeeding. If suvorexant is used, monitor the infant for sedation, especially if the infant is a newborn or preterm. Until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Suvorexant is extensively bound (>99%) to human plasma proteins and does not preferentially distribute into red blood cells. It binds to both human serum albumin and alpha1-acid glycoprotein. |
||
| 参考文献 | |||
| 其他信息 |
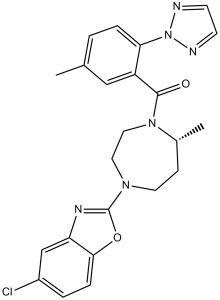
Suvorexant is an aromatic amide obtained by formal condensation of the carboxy group of 5-methyl-2-(2H-1,2,3-triazol-2-yl)benzoic acid with the secondary amino group of 5-chloro-2-[(5R)-5-methyl-1,4-diazepan-1-yl]-1,3-benzoxazole. An orexin receptor antagonist used for the management of insomnia. It has a role as a central nervous system depressant and an orexin receptor antagonist. It is a member of 1,3-benzoxazoles, a member of triazoles, a diazepine, an aromatic amide and an organochlorine compound.
Suvorexant is a DEA Schedule IV controlled substance. Substances in the DEA Schedule IV have a low potential for abuse relative to substances in Schedule III. It is a Depressants substance. Suvorexant is a selective dual antagonist of orexin receptors OX1R and OX2R that promotes sleep by reducing wakefulness and arousal. It has been approved for the treatment of insomnia. Suvorexant is an Orexin Receptor Antagonist. The mechanism of action of suvorexant is as an Orexin Receptor Antagonist, and P-Glycoprotein Inhibitor, and Cytochrome P450 3A Inhibitor. Suvorexant is an orexin receptor antagonist used for the treatment of insomnia and sleep disorders. Suvorexant therapy is associated with rare occurrence of transient serum enzyme elevations, but has not been implicated in cases of clinically apparent liver injury. Suvorexant is an orally bioavailable antagonist of the orexin receptors orexin receptor type 1 (OX1R) and orexin receptor type 2 (OX2R), that can be used for the treatment of insomnia. Upon oral administration, suvorexant targets and binds to the orexin receptors OX1R and OX2R. This blocks the binding of the neuropeptides orexin-A and orexin-B to OX1R and OX2R, and prevents wakefulness that results from orexin signaling. Drug Indication Suvorexant is indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance. FDA Label Mechanism of Action Suvorexant is a dual antagonist of orexin receptors OX1R and OX2R. It exerts its pharmacological effect by inhibiting binding of neuropeptides orexin A and B, also known as hypocretin 1 and 2, that are produced by neurons in the lateral hypothalamus. These neurons control the wake-promoting centers of the brain and are active during wakefulness, especially during motor activities, and stop firing during sleep. By inhibiting the reinforcement of arousal systems, suvorexant use causes a decrease in arousal and wakefulness, rather than having a direct sleep-promoting effect. Despite increased understanding of the biological basis for sleep control in the brain, few novel mechanisms for the treatment of insomnia have been identified in recent years. One notable exception is inhibition of the excitatory neuropeptides orexins A and B by design of orexin receptor antagonists. Herein, we describe how efforts to understand the origin of poor oral pharmacokinetics in a leading HTS-derived diazepane orexin receptor antagonist led to the identification of compound 10 with a 7-methyl substitution on the diazepane core. Though 10 displayed good potency, improved pharmacokinetics, and excellent in vivo efficacy, it formed reactive metabolites in microsomal incubations. A mechanistic hypothesis coupled with an in vitro assay to assess bioactivation led to replacement of the fluoroquinazoline ring of 10 with a chlorobenzoxazole to provide 3 (MK-4305), a potent dual orexin receptor antagonist that is currently being tested in phase III clinical trials for the treatment of primary insomnia.[1] Orexin receptor antagonism represents a novel approach for the treatment of insomnia that directly targets sleep/wake regulation. Several such compounds have entered into clinical development, including the dual orexin receptor antagonists, suvorexant and almorexant. In this study, we have used equilibrium and kinetic binding studies with the orexin-2 (OX₂) selective antagonist radioligand, [³H]-EMPA, to profile several orexin receptor antagonists. Furthermore, selected compounds were studied in cell-based assays of inositol phosphate accumulation and ERK-1/2 phosphorylation in CHO cells stably expressing the OX2 receptor that employ different agonist incubation times (30 and 5 min, respectively). EMPA, suvorexant, almorexant and TCS-OX-29 all bind to the OX₂ receptor with moderate to high affinity (pk(I) values ≥ 7.5), whereas the primarily OX1 selective antagonists SB-334867 and SB-408124 displayed low affinity (pK(I) values ca. 6). Competition kinetic analysis showed that the compounds displayed a range of dissociation rates from very fast (TCS-OX2-29, k(off) = 0.22 min⁻¹) to very slow (almorexant, k(off) = 0.005 min⁻¹). Notably, there was a clear correlation between association rate and affinity. In the cell-based assays, fast-offset antagonists EMPA and TCS-OX2-29 displayed surmountable antagonism of orexin-A agonist activity. However, both suvorexant and particularly almorexant cause concentration-dependent depression in the maximal orexin-A response, a profile that is more evident with a shorter agonist incubation time. Analysis according to a hemi-equilibrium model suggests that antagonist dissociation is slower in a cellular system than in membrane binding; under these conditions, almorexant effectively acts as a pseudo-irreversible antagonist.[2] |
| 分子式 |
C23H23CLN6O2
|
|
|---|---|---|
| 分子量 |
450.9207
|
|
| 精确质量 |
450.16
|
|
| CAS号 |
1030377-33-3
|
|
| 相关CAS号 |
|
|
| PubChem CID |
24965990
|
|
| 外观&性状 |
Typically exists as solid at room temperature
|
|
| LogP |
4.9
|
|
| tPSA |
80.3
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
32
|
|
| 分子复杂度/Complexity |
664
|
|
| 定义原子立体中心数目 |
1
|
|
| SMILES |
C[C@@H]1CCN(CCN1C(=O)C2=C(C=CC(=C2)C)N3N=CC=N3)C4=NC5=C(O4)C=CC(=C5)Cl
|
|
| InChi Key |
JYTNQNCOQXFQPK-MRXNPFEDSA-N
|
|
| InChi Code |
InChI=1S/C23H23ClN6O2/c1-15-3-5-20(30-25-8-9-26-30)18(13-15)22(31)29-12-11-28(10-7-16(29)2)23-27-19-14-17(24)4-6-21(19)32-23/h3-6,8-9,13-14,16H,7,10-12H2,1-2H3/t16-/m1/s1
|
|
| 化学名 |
[(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl]-[5-methyl-2-(triazol-2-yl)phenyl]methanone
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|---|
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.2177 mL | 11.0884 mL | 22.1769 mL | |
| 5 mM | 0.4435 mL | 2.2177 mL | 4.4354 mL | |
| 10 mM | 0.2218 mL | 1.1088 mL | 2.2177 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A multi-center, double-blind, randomized, parallel design study to compare the effectiveness of suvorexant versus placebo on sleep pressure and circadian rhythm in insomniacs with hypertension: The Super 1 study
CTID: UMIN000023389
Phase: Status: Complete: follow-up complete
Date: 2016-08-12