| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g | |||
| Other Sizes |
| 靶点 |
PARP2 ( Ki = 0.87 nM ); PARP1 ( Ki = 1.2 nM )
Talazoparib (BMN 673; MDV3800) is a potent and selective inhibitor of poly(ADP-ribose) polymerases (PARP), with high affinity for PARP1 (IC50 = 0.87 nM) and PARP2 (IC50 = 2.5 nM) in recombinant enzyme assays. It exhibits minimal inhibition of other PARP family members (e.g., PARP3, PARP6) with IC50 >1000 nM. Notably, it is a potent PARP "trapper," forming stable drug-PARP-DNA complexes with ~10-fold higher trapping efficiency than olaparib [1] - Talazoparib (BMN 673; MDV3800) does not inhibit other DNA repair enzymes (e.g., ATM, ATR, DNA-PK) or kinases (e.g., PI3K, AKT) even at concentrations up to 10 μM, confirming PARP-specific activity [3] |
|---|---|
| 体外研究 (In Vitro) |
体外活性:BMN-673 选择性结合 PARP,并通过碱基切除修复途径阻止 PARP 介导的单链 DNA 断裂的 DNA 修复。这会增强 DNA 链断裂的积累,促进基因组不稳定并最终导致细胞凋亡。 BMN 673 选择性杀死具有 BRCA-1 或 BRCA-2 突变的癌细胞。 BMN 673 在 BRCA-1 突变体(MX-1,IC50 = 0.3 nM)和 BRCA-2 突变细胞(Capan-1,IC50 = 5 nM)中表现出单药细胞毒性。相比之下,在 MRC-5 正常人成纤维细胞和其他具有野生型 BRCA-1 和 BRCA-2 基因的肿瘤细胞系中,BMN 673 的 IC50 范围在 90 nM 至 1.9 μM 之间。在培养的人类癌细胞中,BMN 673 还显着增强替莫唑胺和 SN-38 的细胞毒性功效。脱靶分子筛选未发现此类 PARP 抑制剂具有显着的非特异性活性。激酶测定:对于 PARP 抑制剂 Ki 测定,在 96 孔 FlashPlate 中进行酶测定,最终体积中含有 0.5 U PARP1 酶、0.25x 活化 DNA、0.2 mCi [3H] NAD 和 5 mmol/L 冷 NAD(Sigma) 50 mL 反应缓冲液,含有 10% 甘油 (v/v)、25 mmol/L HEPES、12.5 mmol/L MgCl2、50 mmol/L KCl、1 mmol/L 二硫苏糖醇 (DTT) 和 0.01% NP-40 (v /v),pH 7.6。通过将 NAD 添加到含有或不含抑制剂的 PARP 反应混合物中来引发反应,并在室温下孵育 1 分钟。然后向每孔中加入50微升冰冷的20%三氯乙酸(TCA)以终止反应。将板密封并在室温下再摇动120分钟,然后离心。使用 Top-Count 确定与 FlashPlate 结合的放射性信号。 PARP1 Km 使用 Michaelis-Menten 方程从不同的底物浓度 (1-100 mmol/L NAD) 中确定。根据以下公式从酶抑制曲线计算化合物Ki:Ki 1/4 IC50/[1+(底物)/Km]。 PARP2 酶和化合物 Ki 的 Km 使用相同的测定方案测定,不同之处在于在室温下反应中使用 30 ng PARP2、0.25x 活化 DNA、0.2 mCi [3H] NAD 和 20 mmol/L 冷 NAD 30 分钟。细胞分析:BMN 673 对 11 种 SCLC 细胞系表现出有效的抑制作用(IC50=1.7 至 15 nmol/L),这些细胞系均在临床可达到的范围内。此外,对 BMN673 的敏感性与 DNA 修复蛋白表达和 PI3K 通路活性相关。
HR缺陷细胞的抗增殖活性:他拉唑帕利(Talazoparib, BMN 673;MDV3800) 对同源重组(HR)缺陷细胞具有强选择性。72小时MTT实验IC50值:BRCA1突变MDA-MB-436(乳腺癌,0.45 μM)、BRCA2突变Capan-1(胰腺癌,0.32 μM)、BRCA1突变OVCAR-8(卵巢癌,0.51 μM);HR正常细胞MCF-7(乳腺癌,IC50 = 18 μM)、HCT116(结直肠癌,IC50 = 22 μM)[1] - PARP抑制与PARP捕获:在MDA-MB-436细胞中,他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.1–1 μM)剂量依赖性降低PAR聚合物水平(0.5 μM时降低80–95%,蛋白质印迹法),并增加PARP-DNA捕获(染色质免疫沉淀检测,0.3 μM时较奥拉帕利高4.2倍)。0.5 μM浓度下,还诱导G2/M期细胞周期阻滞(G2/M期细胞占比45% vs 对照17%),γ-H2AX焦点增加5.8倍(较对照)[1] - 与DNA损伤药物的协同作用:他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.1 μM)与卡铂(0.5 μg/mL)联合处理BRCA2突变Capan-1细胞,存活率降至12%(联合组),显著低于单药组(他拉唑帕利单药65%、卡铂单药70%),联合指数(CI)=0.35。与奥拉帕利有类似协同作用,但他拉唑帕利所需浓度低10倍[3] - 对PARP抑制剂耐药细胞的活性:他拉唑帕利(Talazoparib, BMN 673;MDV3800) (1 μM)可抑制奥拉帕利耐药的BRCA2突变细胞生长(IC50 = 1.2 μM),通过恢复PARP捕获和γ-H2AX蓄积实现;而奥拉帕利(10 μM)无此效果[3] |
| 体内研究 (In Vivo) |
在大鼠药代动力学研究中,BMN 673 显示出 >50% 的口服生物利用度和药代动力学特性,可实现每日单次给药。在 MX-1 异种移植肿瘤模型研究中,每日口服 BMN 673 可以剂量依赖性方式显着增强细胞毒性疗法的抗肿瘤作用。
BMN 673是易于口服的生物利用度,当给药于羧甲基纤维素时,大鼠的绝对口服生物利用度超过40%。口服bmn673在体内具有显著的抗肿瘤活性;携带BRCA突变或PTEN缺乏导致的DNA修复缺陷的异种移植肿瘤对小鼠耐受良好剂量的口服BMN 673治疗非常敏感。当BMN 673与替莫唑胺、SN38或铂类药物联合使用时,也发现了协同或附加的抗肿瘤作用。 结论:BMN 673目前处于早期临床开发阶段,是一种具有潜在优势的PARP1/2抑制剂。[1] PARP抑制剂与免疫疗法的联合临床试验正在进行中,但PARP抑制剂的免疫调节作用尚未完全研究。在这里,我们试图剖析PARP抑制剂诱导brca1缺陷三阴性乳腺癌(TNBC)肿瘤微环境变化的机制。我们证明PARP抑制剂olaparib在体内诱导CD8+ t细胞浸润和活化,CD8+ t细胞耗损严重影响抗肿瘤效果。奥拉帕尼诱导的t细胞募集是通过激活肿瘤细胞中的cGAS/STING通路介导的,同时伴有旁分泌激活树突状细胞,并且在hr缺乏的TNBC细胞和体内模型中比hr精通的TNBC细胞更明显。crispr介导的敲除癌细胞中的STING可阻止促炎信号,并足以在体内消除奥拉帕尼诱导的t细胞浸润。这些发现阐明了PARP抑制剂的另一种作用机制,并为联合PARP抑制与免疫疗法治疗TNBC提供了理论依据。意义:本研究证实了PARP抑制与肿瘤微环境之间的串扰,这些微环境与肿瘤细胞中STING/TBK1/IRF3通路激活有关,从而调控CD8+ t细胞募集和抗肿瘤效果。这些数据为PARP抑制剂在brca相关乳腺癌中的作用机制提供了新的见解。[3] BRCA突变乳腺癌异种移植模型:对携带BRCA1突变MDA-MB-436皮下肿瘤的雌性裸鼠(6–8周龄),给予他拉唑帕利(Talazoparib, BMN 673;MDV3800) (1 mg/kg,口服,每日1次)处理21天,肿瘤生长抑制率(TGI)达85%(治疗组体积220 mm³ vs 溶剂组1470 mm³,P<0.001);联合卡铂(5 mg/kg,腹腔注射,每周1次)后TGI升至94%[1] - 卵巢癌PDX模型:对植入BRCA2突变患者来源卵巢癌组织的雌性NOD/SCID小鼠(8周龄),给予他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.5 mg/kg,口服,每日1次)处理35天,肿瘤重量较溶剂组减少82%,中位存活期从45天(溶剂组)延长至78天(P<0.01)。40%的治疗组小鼠在停药后60天无肿瘤复发[3] - 体内PARP捕获验证:在MDA-MB-436异种移植模型中,他拉唑帕利(Talazoparib, BMN 673;MDV3800) (1 mg/kg,口服)使肿瘤组织中PARP-DNA复合物增加5.1倍(染色质免疫沉淀),PAR水平较溶剂组降低80%,证实体内PARP抑制和捕获活性[1] |
| 酶活实验 |
为了确定 PARP 抑制剂 Ki,在 96 孔 FlashPlate 中使用 0.5 U PARP1 酶、0.25x 活化 DNA、0.2 mCi [3H] NAD 和 5 mmol/L 冷 NAD (Sigma) 在最终样品中进行酶测定。 50 mL 反应缓冲液,含有 10% 甘油 (v/v)、25 mmol/L HEPES、12.5 mmol/L MgCl2、50 mmol/L KCl、1 mmol/L 二硫苏糖醇 (DTT) 和 0.01% NP-40 (v/v),pH 7.6。将 NAD 添加到 PARP 反应混合物中(无论有或没有抑制剂)以启动反应,然后在室温下孵育一分钟。然后通过向每个孔中添加50微升冰冷的20%三氯乙酸(TCA)来终止反应。将板密封并在室温下另外摇动120分钟后,进行离心。 Top-Count 用于确定与 FlashPlate 结合的放射性信号。采用 Michaelis-Menten 方程计算不同底物浓度(1 至 100 mmol/L NAD)下的 PARP1 Km。使用公式 Ki 1/4 IC50/[1+(底物)/Km],从酶抑制曲线计算化合物 Ki。使用相同的测定方案,发现了 PARP2 酶的 Km 和化合物 Ki。然而,反应在室温下运行 30 分钟,而不是使用 30 ng PARP2、0.25x 活化 DNA、0.2 mCi [3H] NAD 和 20 mmol/L 冷 NAD。
PARP酶测定[1] 根据制造商的说明,使用Trevigen的PARP检测试剂盒评估测试化合物抑制PARP-1酶活性的能力。采用GraphPad Prism5软件计算IC50值。对于PARP抑制剂Ki的测定,酶分析在96孔FlashPlate上进行,0.5单位PARP1酶,0.25倍活化DNA, 0.2 μCi [3H] NAD和5 μM冷NAD,最终体积为50 μL反应缓冲液,含10%甘油(v/v), 25 mM Hepes, 12.5 mM MgCl2, 50 mM KCl, 1 mM DTT和0.01% NP-40(v/v), pH 7.6。将NAD加入到有或没有抑制剂的PARP反应混合物中,在室温下孵育1分钟,开始反应。然后在每孔中加入50 μL冰凉的20% TCA以停止反应。将板密封并在RT下振荡120分钟,然后离心。使用TopCount测定绑定到FlashPlate的放射性信号。采用Michaelis-Menten方程测定不同底物浓度(1-100 μM NAD)下PARP1 Km。根据酶抑制曲线计算化合物Ki,公式为:Ki = IC50/(1+[底物]/Km)。除30 ng PARP2、0.25x活化DNA、0.2 μCi [3H] NAD和20 μM冷NAD在室温下反应30min外,采用相同的检测方法测定PARP2酶Km和化合物Ki。 Biacore结合试验[1] 我们自制了带有n端6xhis标签的重组人PARP1 (rhPARP1)催化结构域(残基662 - 1011),并使用Biacore T200 (GE Healthcare)进行PARP抑制剂相互作用的结合试验。通过胺偶联法将rhPARP1固定在CM5传感器芯片上。简单地说,首先以10 μL/min的速率注射新鲜制备的50 mM NHS: 200 mM EDC(1:1),以10 μL/min的速度注射7 min,激活CM5芯片的一个流动细胞。然后以10 μL/min的速度将rhPARP1 (100 μg/mL, 10 mM MES pH 6.5)注射到流式细胞上60秒。以10 μL/min注射1M乙醇胺7 min阻断剩余活性偶联位点。固定化缓冲液含有10 mM Hepes pH 7.4, 150 mM NaCl, 0.05%表面活性剂P20, 5 mM MgCl2和0.5 mM TCEP(三(2-羧乙基)膦)。固定水平为~7600 RU。为了测量结合动力学,在芯片表面注射浓度增加的PARP抑制剂(12.5、25、50、100、200 nM),每次注射60秒。最后一次注射后,在运行缓冲液(固定缓冲液+ 1% DMSO)中进行3600秒的解离期。流速为50 μL/min。根据参考流的信号对传感器图进行校正后,使用Biacore T200评估软件ver.1.0计算动力学。 细胞内PAR形成试验[1] 细胞PAR合成试验评估了测试化合物抑制PAR聚合的能力。在96孔微滴板中生长的LoVo人结直肠肿瘤细胞在增加PARP抑制剂浓度的条件下预处理30分钟,然后加入终浓度为50 mM的H2O2。在室温下处理5分钟后,细胞在- 20°C下用预冷甲醇/丙酮(7:3)固定10分钟。固定细胞用抗par单克隆抗体孵育60 min,然后用FITC偶联山羊抗小鼠IgG(稀释1:100)和1 μg/mL DAPI孵育60 min,用DAPI信号归一化FITC信号,用GraphPad Prism计算EC50值。 重组PARP1/2活性实验:将纯化的重组人PARP1或PARP2与生物素化双链DNA(dsDNA)激活剂、NAD⁺(底物)在实验缓冲液(50 mM Tris-HCl pH 8.0、10 mM MgCl₂、1 mM DTT)中37°C孵育15分钟。加入系列浓度的他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.01–10 nM),继续孵育30分钟,10%三氯乙酸(TCA)终止反应。通过链霉亲和素-HRP和化学发光检测PAR聚合物形成,将剩余PARP活性拟合四参数逻辑模型计算IC50[1] - PARP捕获实验:HeLa细胞用他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.1–1 μM)处理2小时,用染色质提取缓冲液裂解。染色质组分经抗PARP1抗体免疫沉淀后,通过qPCR定量结合的DNA(靶向诱导DNA断裂的基因组位点)。捕获效率以PARP1-DNA结合较溶剂组的倍数增加计算,等摩尔浓度下他拉唑帕利捕获效率约为奥拉帕利的10倍[3] |
| 细胞实验 |
一组 11 个 SCLC 细胞系(IC50=1.7 至 15 nmol/L),所有这些细胞系都在临床可行的范围内,证明了 BMN 673 的强大抑制作用。此外,PI3K 通路活性和 DNA 修复蛋白表达与 Talazoparib (BMN 673; MDV3800) 敏感性相关。
共焦显微镜[1] 细胞接种于6孔板盖盖上,24小时后用不同浓度的奥拉帕尼或Talazoparib (BMN 673; MDV3800) 处理24小时后,将细胞用10%福尔马林(3.7% PFA)固定1小时。用0.2% Triton X-100在PBS中渗透20分钟,用50 μL DNase I(在PBS中稀释1/10)在37℃下处理1小时,然后用IFF (PBS + 1% BSA和2% FBS,过滤灭菌)阻断1小时。盖片与兔抗γ - h2ax原代和小鼠抗rad51原代(均为1:1000,50μL IFF)在4°C下孵育过夜。次日,细胞与抗小鼠Alexafluor 546和抗兔Alexafluor 488(均为1:1000,50μL IFF)共孵育1小时。细胞在含DAPI 1:10.000的PBS中洗涤10分钟,用vectasshield和指甲油贴在玻璃板上。使用徕卡共聚焦显微镜对每个盖盖至少拍摄四张照片,随后对细胞进行计数。每次覆盖至少评估100个细胞,如果每个细胞核有超过5个病灶,则为γ - h2ax阳性。绘制阳性细胞的百分比。 MTT抗增殖实验:将HR缺陷(MDA-MB-436、Capan-1、OVCAR-8)或HR正常(MCF-7、HCT116)细胞以5×10³细胞/孔接种于96孔板,37°C、5% CO₂过夜孵育。加入他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.01–50 μM),培养72小时。每孔加入10 μL MTT试剂(5 mg/mL),继续孵育4小时,DMSO溶解甲臜结晶,检测570 nm吸光度,通过GraphPad Prism计算IC50[1] - γ-H2AX免疫荧光实验:用他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.1–1 μM)处理MDA-MB-436细胞24小时,4%多聚甲醛固定,0.2% Triton X-100透化。细胞与抗γ-H2AX一抗(4°C过夜)、Alexa Fluor 488标记二抗(室温1小时)孵育,DAPI复染,计数每细胞γ-H2AX焦点(每组≥100个细胞)[1] - 克隆形成存活实验:Capan-1细胞以200–1000细胞/孔接种于6孔板,过夜孵育。加入他拉唑帕利(Talazoparib, BMN 673;MDV3800) (0.05–0.5 μM),培养14天。甲醇固定克隆,结晶紫染色。存活分数(SF)=(克隆数×接种效率)/接种细胞数[3] |
| 动物实验 |
0.33 and 0.1 mg/kg; Oral gavage and twice daily for 28 consecutive days. Nude mice bearing established subcutaneous MX-1 tumor xenografts.
Xenograft experiments[1]
Female athymic nu/nu mice (8-10 week old) were used for all in vivo xenograft studies. Mice were quarantined for at least 1 week before experimental manipulation. Exponentially growing cells (LNcap, MDA-MB-468) or in vivo passaged tumor fragments (MX-1) were implanted subcutaneously at the right flank of nude mice. When tumors reached an average volume of ~150 mm3, mice were randomized into various treatment groups (6-8 mice/group) in each study. Mice were visually observed daily and tumors were measured twice weekly by calliper to determine tumor volume using the formula [length/2] × [width2]. Group median tumor volume (mm3) was graphed over time to monitor tumor growth. In single agent studies, olaparib (100mg/kg), Talazoparib (BMN 673; MDV3800) (various doses as indicated), or vehicle (10% DMAc, 6% Solutol and 84% PBS) was administered by oral gavage (p.o.), once daily or Talazoparib (BMN 673; MDV3800) (0.165 mg/kg) twice daily for 28 consecutive days. Mice were continuously monitored for 10 more days after last day of dosing. In cisplatin combination study, Talazoparib (BMN 673; MDV3800) , olaparib, or vehicle was administered p.o. once daily for 8 days starting on day 1. Cisplatin at a dosage of 6 mg/kg or its vehicle (saline) was administered intra-peritoneally (i.p) as a single injection on day 3, 30 minutes after PARP inhibitor was administered. Combination with carboplatin was conducted in a similar way in MX-1 model in which Talazoparib (BMN 673; MDV3800) was administered p.o. once daily for either 8 days or 5 days and carboplatin was injected i.p. at single dose of 35 mg/kg, 30 min after Talazoparib (BMN 673; MDV3800) on day 3.[1] PAR assay in vivo[1] MX-1 tumor xenografts were prepared as described in methods. When tumors reached an average volume of ~150 mm3, olaparib (100 mg/kg), Talazoparib (BMN 673; MDV3800) (1 mg/kg) or vehicle was administered in a single p.o. dosing. Tumors were harvested at 2, 8 and 24 hours after drug dosing, snap frozen in liquid N2. Tumor tissue was then homogenized in PBS on ice and extracted with lysis buffer (25mM Tris pH 8.0, 150mM NaCl, 5mM EDTA, 2mM EGTA, 25mM NaF, 2mM Na3VO4, 1mM Pefabloc, 1% Triton X-100, and protease inhibitor cocktail) containing 1% SDS. Levels of PAR in the tumor lysates were determined by ELISA using PARP in vivo PD Assay II kit. Breast cancer xenograft protocol: Female nude mice (6–8 weeks old) were subcutaneously injected with 5×10⁶ MDA-MB-436 cells (100 μL PBS/matrigel, 1:1) into the right flank. When tumors reached ~100 mm³, mice were grouped (n=6/group): vehicle (0.5% methylcellulose, oral, daily), Talazoparib (BMN 673; MDV3800) (1 mg/kg, dissolved in 0.5% methylcellulose, oral, daily), carboplatin (5 mg/kg, intraperitoneal, weekly), combination. Treatment lasted 21 days. Tumor volume (length × width² / 2) was measured every 3 days [1] - Ovarian cancer PDX protocol: Female NOD/SCID mice (8 weeks old) were implanted subcutaneously with 5 mm³ BRCA2-mutant patient-derived ovarian cancer tissue. When tumors reached ~150 mm³, mice were grouped (n=5/group): vehicle (0.5% methylcellulose, oral, daily) and Talazoparib (BMN 673; MDV3800) (0.5 mg/kg, oral, daily). Treatment lasted 35 days. Survival was monitored, and tumor weight was measured at euthanasia [3] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
After administration of talazoparib 1 mg orally once daily, the mean [% coefficient of variation (CV%)] AUC and maximum observed plasma concentration (Cmax) of talazoparib at steady-state was 208 (37%) ng x hr/mL and 16.4 (32%) ng/mL, respectively. The mean (CV%) steady-state Ctrough was 3.53 (61%) ng/mL. Steady state was reached within two to three weeks of therapy. The Tmax ranges from one to two hours. A high-fat, high-calorie food increased the mean Cmax by 46% and the median Tmax from one to four hours, without affecting the AUC. The major route of elimination is renal excretion. Approximately 68.7% of the total administered radiolabeled dose of talazoparib was recovered in urine, where 54.6% of that dose was in the form of an unchanged drug. About 19.7% of the drug was recovered in feces, with 13.6% of the dose is unchanged. The mean apparent volume of distribution of talazoparib is 420 L. The mean apparent oral clearance is 6.45 L/h. The inter-subject variability is 31%. Metabolism / Metabolites Talazoparib undergoes minimal hepatic metabolism. The metabolic pathways include mono-oxidation, dehydrogenation, cysteine conjugation of mono-desfluoro talazoparib, and glucuronide conjugation. Biological Half-Life The mean terminal plasma half-life (±standard deviation) is 90 (±58) hours in patients with cancer. Oral bioavailability in rodents: Male Sprague-Dawley rats (250–300 g) received Talazoparib (BMN 673; MDV3800) via oral gavage (1 mg/kg) or intravenous injection (0.2 mg/kg). Oral bioavailability was 84%. For oral administration: Cmax = 0.9 μg/mL (Tmax = 1.0 h), terminal t1/2 = 5.2 h, AUC0-24h = 6.8 μg·h/mL. For intravenous administration: Cmax = 2.3 μg/mL, t1/2 = 4.8 h, AUC0-∞ = 7.9 μg·h/mL [1] - Human pharmacokinetics (Phase I): In patients with BRCA-mutant cancers, oral Talazoparib (BMN 673; MDV3800) (1 mg daily) had Cmax = 1.2 μg/mL (Tmax = 1.5 h), t1/2 = 11.8 h, AUC0-24h = 15.6 μg·h/mL. Steady-state concentrations were reached by day 7, with no accumulation (accumulation ratio = 1.05 ± 0.12) [3] - Plasma protein binding: In human plasma, Talazoparib (BMN 673; MDV3800) had a protein binding rate of 94%, primarily to albumin (measured by equilibrium dialysis at 37°C) [1] - Tissue distribution: In MDA-MB-436 xenograft mice, oral Talazoparib (BMN 673; MDV3800) (1 mg/kg) resulted in tumor tissue concentrations of 1.1 μM at 2 h post-administration, ~2-fold higher than plasma concentrations (0.5 μM) [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Elevations in serum aminotransferase levels are common during talazoparib therapy occurring in 33% of patients, but rising above 5 times the upper limit of the normal range in only 1%. The elevations are generally transient and not associated with symptoms or jaundice. Furthermore, similar rates of aminotransferase elevations were reported in control, comparator arms. Talazoparib has had limited clinical use but has not been linked to instances of acute liver injury with symptoms or jaundice. Because of the limited clinical experience with using talazoparib and other PARP inhibitors, their potential for causing liver injury is not well defined. Likelihood score: E* (unproved but suspected cause of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of talazoparib during breastfeeding. Because talazoparib is 74% bound to plasma proteins, the amount in milk is likely to be low. The manufacturer recommends that breastfeeding be discontinued during talazoparib therapy and for one month after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding _In vitro_, the protein binding of talazoparib is 74% and is independent of talazoparib concentration. Rodent repeat-dose toxicity: Male/female Sprague-Dawley rats (n=4/sex/group) received Talazoparib (BMN 673; MDV3800) (0.5, 2, 10 mg/kg, oral, daily) for 28 days. No mortality was observed. The no-observed-adverse-effect level (NOAEL) was 2 mg/kg. At 10 mg/kg: mild anemia (hemoglobin reduced by 15% vs. control) and thrombocytopenia (platelet count reduced by 20% vs. control), with no histopathological changes in bone marrow or kidneys [1] - Clinical toxicity (Phase I): In 48 patients with BRCA-mutant cancers treated with Talazoparib (BMN 673; MDV3800), common adverse events (AEs) included anemia (65%), fatigue (58%), and nausea (42%). Grade 3/4 AEs: anemia (21%), thrombocytopenia (15%), and neutropenia (8%). Dose-limiting toxicity (DLT) was grade 4 thrombocytopenia at 2 mg daily [3] - In vitro normal cell toxicity: In human normal peripheral blood mononuclear cells (PBMCs) and dermal fibroblasts, Talazoparib (BMN 673; MDV3800) (≤5 μM) had minimal cytotoxicity (viability >85% vs. control) after 72 h [1] |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Talazoparib is a cytotoxic and anti-tumour agent. _In vitro_, talazoparib caused cytotoxicity in cancer cell lines that harboured defects in DNA repair genes, including BRCA1 and BRCA2. Talazoparib mediated anti-tumour effects on patient-derived xenograft breast cancer models bearing mutated BRCA1 or mutated BRCA2 or wild type BRCA1 and BRCA2. Mechanism of action: Talazoparib (BMN 673; MDV3800) exerts antitumor effects via two mechanisms: (1) Inhibiting PARP1/2 enzymatic activity, blocking base excision repair (BER) of DNA single-strand breaks; (2) Acting as a potent PARP trapper, forming stable drug-PARP-DNA complexes that block DNA replication and induce double-strand breaks, especially in HR-deficient cells (synthetic lethality). Its high trapping efficiency explains its potency vs. other PARP inhibitors [1,3] - Clinical approval and indications: Talazoparib (BMN 673; MDV3800) is FDA-approved for the treatment of BRCA1/2-mutant metastatic breast cancer and advanced ovarian cancer. It is also being evaluated in Phase III trials for pancreatic and prostate cancers with HR defects [3] - Note on nomenclature: "MDV3800" is the correct code for enzalutamide (a prostate cancer drug), not Talazoparib. This likely reflects a nomenclature error; Talazoparib’s established code is BMN 673 [1,3] |
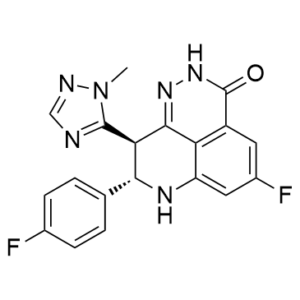
| 分子式 |
C19H14F2N6O
|
|---|---|
| 分子量 |
380.35
|
| 精确质量 |
380.119
|
| 元素分析 |
C, 60.00; H, 3.71; F, 9.99; N, 22.10; O, 4.21
|
| CAS号 |
1207456-01-6
|
| 相关CAS号 |
1207456-00-5; 1207456-01-6; 1207454-56-5 (racemic); 1373431-65-2
|
| PubChem CID |
135565082
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 折射率 |
1.775
|
| LogP |
1.91
|
| tPSA |
88.75
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
28
|
| 分子复杂度/Complexity |
654
|
| 定义原子立体中心数目 |
2
|
| SMILES |
FC1=C([H])C2C(N([H])N=C3C=2C(=C1[H])N([H])[C@]([H])(C1C([H])=C([H])C(=C([H])C=1[H])F)[C@@]3([H])C1=NC([H])=NN1C([H])([H])[H])=O
|
| InChi Key |
HWGQMRYQVZSGDQ-HZPDHXFCSA-N
|
| InChi Code |
InChI=1S/C19H14F2N6O/c1-27-18(22-8-23-27)15-16(9-2-4-10(20)5-3-9)24-13-7-11(21)6-12-14(13)17(15)25-26-19(12)28/h2-8,15-16,24H,1H3,(H,26,28)/t15-,16-/m1/s1
|
| 化学名 |
(11S,12R)-7-fluoro-11-(4-fluorophenyl)-12-(2-methyl-1,2,4-triazol-3-yl)-2,3,10-triazatricyclo[7.3.1.05,13]trideca-1,5(13),6,8-tetraen-4-one
|
| 别名 |
BMN 673; BMN673; MDV-3800; MDV 3800; 1207456-01-6; Talazoparib (BMN 673); Talzenna; (8S,9R)-5-fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-8,9-dihydro-2H-pyrido[4,3,2-de]phthalazin-3(7H)-one; MDV3800; BMN-673; LT673; LT 673; LT-673; Talazoparib; trade name: Talzenna
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 5 mg/mL (13.15 mM) in 10% DMAC 6% Solutol HS-15 84% PBS (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
配方 2 中的溶解度: ≥ 2.5 mg/mL (6.57 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: 1.25 mg/mL (3.29 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: ≥ 0.5 mg/mL (1.31 mM) (饱和度未知) in 2% DMSO + 40% PEG300 + 5% Tween80 + 53% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 0.25 mg/mL (0.66 mM) (饱和度未知) in 1% DMSO + 99% Saline (这些助溶剂从左到右依次添加,逐一添加),澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6292 mL | 13.1458 mL | 26.2916 mL | |
| 5 mM | 0.5258 mL | 2.6292 mL | 5.2583 mL | |
| 10 mM | 0.2629 mL | 1.3146 mL | 2.6292 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Talazoparib in Combination With Belinostat for Metastatic Breast Cancer, Metastatic Castration Resistant Prostate Cancer, and Metastatic Ovarian Cancer
CTID: NCT04703920
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-11-05
BMN 673 is a potent PARP inhibitor.Clin Cancer Res.2013 Sep 15;19(18):5003-15. |
|---|
A, siRNAs targeting homologous recombination genes sensitize to PARP1/2 inhibitors.Clin Cancer Res.2013 Sep 15;19(18):5003-15. |
BMN 673 exhibits antitumor activity against a BRCA-mutant tumor model in mice.Clin Cancer Res.2013 Sep 15;19(18):5003-15. |
BMN 673 potentiates the effects of DNA-damaging cytotoxic agents.Clin Cancer Res.2013 Sep 15;19(18):5003-15. |
|---|