| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|

| 靶点 |
HIV-1/2 nucleotide reverse transcriptase
|
|---|---|
| 体外研究 (In Vitro) |
富马酸替诺福韦艾拉酚胺(GS-7340 富马酸盐)在所有细胞类型中均表现出相当的抗病毒活性,对于 MT-4 和 MT-2 细胞,CC50 范围分别为 4.7 至 42 μM。富马酸盐的浓度范围为 5 至 7 nM。一组 HIV-1 和 HIV-2 分离株(包括 HIV-1 M A 至 G 亚型以及 N 和 O 组分离株)用于评估 TAF 的抗病毒活性。在 PBMC 中测试的 29 个主要 HIV-1 分离株中,发现 TAF 的平均 EC50 为 3.5 nM,而内部对照 AZT 的平均 EC50 为 11.8 nM。对于 HIV-2 分离株,AZT 的平均 EC50 为 6.4 nM,TAF 的平均 EC50 为 1.8 nM [2]。
|
| 体内研究 (In Vivo) |
替诺福韦的酰胺化前药称为 GS-7340 富马酸盐,或替诺福韦艾拉酚胺富马酸盐。与富马酸替诺福韦二吡呋酯(TDF)相比,它具有更好的血浆稳定性和良好的口服生物利用度[1]。
|
| 酶活实验 |
肠道和肝脏S9稳定性[1]
GS-7340与狗或人的肠道和肝脏S9组分在37°C下以96孔板型在10μM下孵育120分钟。在添加化合物后的指定时间点,用9倍体积的含有25%乙腈和50%甲醇的水溶液淬灭样品。将板在3000g下离心30分钟,通过LC-MS/MS分析10μL所得溶液。数据(分析物与内标峰面积比)以半对数标度绘制,并使用指数拟合进行拟合。假设一级动力学,确定了半衰期和代谢速率。使用充分搅拌的肝脏清除模型,通过报告的方法根据半衰期计算预测的肝脏提取量。[1] 血浆稳定性[1] GS-7340在37°C下在狗或人血浆中以2μM孵育4小时。在指定的时间点,通过加入9倍体积的100%乙腈和内标来淬灭孵育的等分试样。最后一次收集后,将样品在3000g下离心30分钟,将上清液转移到含有等体积水的新板上,通过液相色谱法结合三重四极杆质谱法(LC-MS/MS)进行分析。 |
| 细胞实验 |
Caco-2渗透性[1]
如前所述,使用接种在12孔板中的人结肠癌细胞系caco-2的融合单层进行了双向渗透性研究。研究了浓度(10、100或1000μM)或外排转运体抑制对GS-7340渗透的影响。在运输缓冲液中用10μM环孢菌素a(CsA)预孵育细胞单层30分钟以使转运蛋白结合位点饱和后,评估了包括P-糖蛋白(Pgp)在内的外排转运蛋白的抑制效果。预孵育后,加入含有CsA和GS-7340的新鲜测定缓冲液,开始测定。每次测定均进行两次,并测定对照化合物(阿替洛尔、普萘洛尔和地高辛)的渗透性,以满足每批测定板的验收标准。 |
| 动物实验 |
Animals[1]
Male beagle dogs between the ages of 6 and 18 months were used for the in life portion of this study. The animals were housed in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care and were receiving a standard commercial diet. Animals were handled in strict accordance with the Guide for the Care and Use of Laboratory Animals, and the protocol was reviewed by the Institutional Animal Care and Use Committee at SRI International. Animals were between 7 and 11 kg at dosing.[1] Drug Administration[1] For intravenous administration, GS-7340 was formulated in 5% dextrose in water. For oral administration, GS-7340 was formulated in 50 mM citric acid (pH 5.0) at doses of 2 to 10 mg/kg. For the 20 mg/kg dose, GS-7340 was formulated in 50 mM citric acid (pH 5.5) with 0.1% Polysorbate 20. To test the effect of efflux transport inhibition, dogs were administered 2 mg/kg GS-7340 1 h following pretreatment with a 75 mg capsule of CsA.[1] Plasma and PBMC Sample Collection[1] Blood (approximately 0.5 mL) was collected at specified time points over 24 h from the jugular vein (intraportal vein infusion and oral pharmacokinetic studies), the jugular and portal veins (oral administration to portal vein cannulated dogs), or the cephalic vein (jugular vein infusion). Plasma was isolated in Vacutainer tubes containing EDTA as an anticoagulant by centrifugation. At select time points (1, 4, 8, and 24 h postdose) in the 5 mg/kg oral pharmacokinetic study, 8 to 10 mL of blood was collected into Vacutainer cell preparation tubes (Becton Dickinson) for isolation of peripheral blood mononuclear cells (PBMC) and processed following manufacturer instructions. Small aliquots (10 μL) of isolated PBMC pellets diluted in 0.9% NaCl were maintained at room temperature and used to determine cell count. 500 μL of 70% methanol was added to the remaining PBMC pellets and, together with plasma samples, stored at −80 °C until shipment for further processing and analysis. |
| 参考文献 |
|
| 其他信息 |
Tenofovir alafenamide is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic hepatitis B virus infection (HBV) in adults and children 6 years of age and older who weigh at least 55 lb (25 kg) and who meet certain requirements, as determined by a health care provider.
HBV can be an opportunistic infection (OI) of HIV. Drug Indication Vemlidy is indicated for the treatment of chronic hepatitis B (CHB) in adults and paediatric patients 6 years of age and older weighing at least 25 kg (see section 5. 1). |
| 分子式 |
C25H33N6O9P
|
|---|---|
| 分子量 |
593.55
|
| 精确质量 |
592.204
|
| 元素分析 |
C, 50.68; H, 5.61; N, 14.18; O, 24.30; P, 5.23
|
| CAS号 |
379270-38-9
|
| 相关CAS号 |
Tenofovir alafenamide;379270-37-8; 379270-38-9 (fumarate); 1392275-56-7 (hemifumarate);
|
| PubChem CID |
68516365
|
| 外观&性状 |
White to off-white solid powder
|
| LogP |
3.656
|
| tPSA |
227.89
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
14
|
| 可旋转键数目(RBC) |
14
|
| 重原子数目 |
41
|
| 分子复杂度/Complexity |
799
|
| 定义原子立体中心数目 |
3
|
| SMILES |
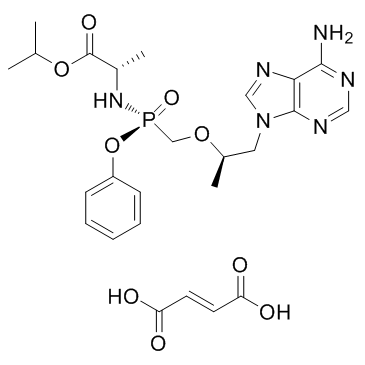
[P@](C([H])([H])O[C@]([H])(C([H])([H])[H])C([H])([H])N1C([H])=NC2=C(N([H])[H])N=C([H])N=C12)(N([H])[C@]([H])(C(=O)OC([H])(C([H])([H])[H])C([H])([H])[H])C([H])([H])[H])(=O)OC1C([H])=C([H])C([H])=C([H])C=1[H].O([H])C(/C(/[H])=C(\[H])/C(=O)O[H])=O
|
| InChi Key |
MEJAFWXKUKMUIR-WIUYAKJJSA-N
|
| InChi Code |
InChI=1S/C21H29N6O5P.C4H4O4/c1-14(2)31-21(28)16(4)26-33(29,32-17-8-6-5-7-9-17)13-30-15(3)10-27-12-25-18-19(22)23-11-24-20(18)27;5-3(6)1-2-4(7)8/h5-9,11-12,14-16H,10,13H2,1-4H3,(H,26,29)(H2,22,23,24);1-2H,(H,5,6)(H,7,8)/b;2-1+/t15-,16+,33?;/m1./s1
|
| 化学名 |
(S)-isopropyl 2-(((S)-((((R)-1-(6-amino-9H-purin-9-yl)propan-2-yl)oxy)methyl)(phenoxy)phosphoryl)amino)propanoate fumarate
|
| 别名 |
GS734; GS-734; GS 734; GS 7340; GS-7340; GS7340; TAF; Tenofovir alafenamide fumarate; GS-7340 fumarate; 379270-38-9; GS-7340 monofumarate; H2S5S51WW6; GS-7340 (fumarate); GS-7339 monofumarate; L-Alanine, N-((S)-(((1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy)methyl)phenoxyphosphinyl)-, 1-methylethyl ester, (2E)-2-butenedioate (1:1); trade name: Genvoya.
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 36 mg/mL (~60.76 mM)
H2O : ≥ 25 mg/mL (~42.19 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 6.67 mg/mL (11.26 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。 (<60°C).
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.6848 mL | 8.4239 mL | 16.8478 mL | |
| 5 mM | 0.3370 mL | 1.6848 mL | 3.3696 mL | |
| 10 mM | 0.1685 mL | 0.8424 mL | 1.6848 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。