| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
They are efficiently absorbed from intestine, and presumably there is some absorption across the skin and lung. /Urea-, uracil- and triazine-based herbicides/ Absorbed through both foliage and roots. It appears to penetrate foliage rapidly, minimizing removal from foliage by rain. /It is/ translocated acropetally through xylem from roots and foliage, accumulating in apical meristems. In mammals, following oral admin, 73-85% is eliminated in metabolized form in feces within 24 hr. Metabolism / Metabolites Terbutryn ... was metabolized by both rats and goats after a single oral dose by one or more of the following pathways: S-demethylation, conversion of thiomethyl into hydroxyl, N-de-ethylation, oxidation of the terminal carbon of the ethyl group to a carboxylic acid, oxidation of a terminal carbon of the t-butyl group to an alcohol or a carboxylic acid, or conjugation with glucuronic acid. Carbon-labeled terbutryn was admin as single oral doses to rats and goats. Urine was collected at intervals up to 72 hr and then analyzed ... after isolation of glucuronides by chromatographic procedures. Five conjugates isolated and identified were: 2-amino-4-(t-butylamino)-6-(S-glucuronyl)-s-triazine; 2-(t-butylamino)-4-ethylamino-6-(S-glucuronyl)-s-triazine; 2-ethyl-amino-(2-methyl)glucuronylpropyl)amino-6-(S-methylthio)-s-triazine; 2-amino-4-(2-(1-glucuronyl-2-methylpropyl)amino)-6-methylthio-s-triazine; 2-ethylamino-4-(2-(2-methyl propan-1-olyl)amino)-6-(S-glucuronyl)-s-triazine. After administration of terbutryne to rats, urinary metabolites observed ... included: 2-hydroxy terbutryne; 2-amino-4-hydroxy-6-t-butylamino-s-triazine; 2-amino-4-t-butylamino-6-mercapto-s-triazine; two S-glucuronides and two t-butyl-O-glucuronides. Other metabolites were formed by one or a combination of the following reactions: N-alkyl oxidation to alcohols or acids: S-demethylation; N-deethylation; and disulfide formation. Microsomes prepared from livers from 30 to 70 year old patients undergoing liver resection were incubated with 6.3 to 1,000 uM atrazine, terbuthylazine, terbutryne, or ametryne , and the incubation mixtures were analyzed for metabolites. The compounds produced a variety of metabolites indicative of S-oxidation, N-dealkylation, and side chain C-oxidation. The metabolites were formed by processes showing biphasic kinetics, Michaelis constants for the first and second phases varying from 1.4 to 20 uM and from 54 to 530 uM, respectively. Atrazine, terbuthylazine, ametryne, or terbutryne at 25 uM was incubated with human liver microsomes containing substrates for cytochrome-P4501A2 (CYP1A2), cytochrome-P4502A6, cytochrome-P4502D6, cytochrome-P4502C9, cytochrome-P4502C19, cytochrome-P4502E1, or cytochrome-P4503A4 (CYP3A4) isozymes. Other microsomal preparations were incubated with 25 or 600 uM of the S-triazines in the presence or absence of alpha-naphthoflavone (aNF), furafylline, quinidine, sulfaphenazole, diethyl-dithiocarbamate, gestodene, or ketoconazole, inhibitors of various specific cytochrome-P450 (P450) isozymes, at concentrations 5 to 10 times greater than their inhibition constants. Microsomal preparations containing substrates for CYP1A2 and CYP3A4 showed the best correlation with the rates of metabolism of the S-triazines. Only aNF and furafylline, inhibitors of CYP1A2, inhibited metabolism of the S-triazines. A human liver microsomal preparation with demonstrated high levels of flavin containing monooxygenase (FMO) activity and purified recombinant human FMO-3 were incubated with ametryne and terbutryne. The extent of sulfoxidation of the two compounds was determined. No significant formation of sulfoxide metabolites was detected, indicating that the FMO system was not involved in the metabolism of S- triazines by human liver microsomes. The authors conclude that these results clearly identify CYP1A2 as the major phase-I P450 isozyme that is involved in the metabolism of S-triazines by human liver microsomes. For more Metabolism/Metabolites (Complete) data for TERBUTRYNE (7 total), please visit the HSDB record page. Terbutryn has known human metabolites that include Terbutrynsulfoxide, t-Butylhydroxy-terbutryn, and 2-Hydroxyethylterbutryn. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Data
LC50 (rat) > 8,000 mg/m3/4h Non-Human Toxicity Values LD50 Rabbit dermal >2,000 mg/kg LD50 Rat oral 2450-2500 mg/kg LD50 Rat oral 2045 mg/kg LC50 Rat inhalation >8 mg/L/4 hr /80% formulation/ For more Non-Human Toxicity Values (Complete) data for TERBUTRYNE (8 total), please visit the HSDB record page. |
| 参考文献 |
Environ Sci Technol. 2014;48(1):244-54.
|
| 其他信息 |
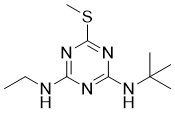
Terbutryn is a methylthio-1,3,5-triazine that is 2-(methylsulfanyl)-1,3,5-triazine substituted by a tert-butylamino and an ethylamino group at positions 2 and 4 respectively. It has a role as a herbicide, a xenobiotic and an environmental contaminant. It is a methylthio-1,3,5-triazine and a diamino-1,3,5-triazine.
Mechanism of Action ... Their chief mode of action appears to involve carbohydrate metabolism. The chlorinated s-triazines inhibit starch accumulation by blocking the prodn of sugars. Similar behavior has been shown for the methoxy & methylthio-s-triazines. It has been reported that the s-triazines affect the tricarboxylic acid cycle with activation of phospho-phenyl pyruvate-carboxylase causing the disappearance of sucrose & glyceric acid with the formation of aspartic & malic acids. /S-triazines/ Inhibition of photosynthesis by disruption of light reactions and blockade of electron transport is the mechanism of action of the 1,3,5-triazine herbicides. /1,3,5-Triazines, from table/ The influence of some s-triazine herbicides on acid phosphatase and phosphodiesterase from corn (Zea mays) roots were investigated. Terbutryn stimulated both phosphatases, whereas prometryn stimulated only the phosphodiesterase. Atrazine desmetryn, prometon, and simazine inhibited acid phosphatase. No effect was exerted by ametryn. The enzyme assays and the kinetic parameters demonstrated that the interferences observed were due to an action on the synthesis of one or both enzymes rather than on the enzyme reactions. The types of the N-alkyl and the chlorine-subsitutuent groups in the structures of the s-triazines tested appear important in determing the degree of the interference. |
| 分子式 |
C10H19N5S
|
|---|---|
| 分子量 |
241.35636
|
| 精确质量 |
241.136
|
| CAS号 |
886-50-0
|
| 相关CAS号 |
Terbutryn-d5;1219804-47-3
|
| PubChem CID |
13450
|
| 外观&性状 |
WHITE, CRYSTALLINE
White powder |
| 密度 |
1.45
|
| 沸点 |
154-160°C
|
| 熔点 |
104-105°C
|
| 闪点 |
2 °C
|
| 折射率 |
1.55
|
| LogP |
2.381
|
| tPSA |
88.03
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
16
|
| 分子复杂度/Complexity |
206
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1C(NCC)=NC(NC(C)(C)C)=NC=1SC
|
| InChi Key |
IROINLKCQGIITA-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C10H19N5S/c1-6-11-7-12-8(15-10(2,3)4)14-9(13-7)16-5/h6H2,1-5H3,(H2,11,12,13,14,15)
|
| 化学名 |
2-N-tert-butyl-4-N-ethyl-6-methylsulfanyl-1,3,5-triazine-2,4-diamine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~414.32 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.36 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.36 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1432 mL | 20.7159 mL | 41.4319 mL | |
| 5 mM | 0.8286 mL | 4.1432 mL | 8.2864 mL | |
| 10 mM | 0.4143 mL | 2.0716 mL | 4.1432 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。