| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Systemic absorption of anaesthetic from the combination cream is directly related to the duration and surface area of application. Although peak plasma concentrations for lidocaine were measured, plasma levels for tetracaine could not be determined due to low levels (<0.9 ng/mL) Tetracaine is rapidly hydrolyzed in the plasma; therefore, volume of distribution could not be determined. Tetracaine is hydrolyzed rapidly in the plasma; therefore, clearance has not been determined. Metabolism / Metabolites Tetracaine is rapidly hydrolyzed by plasma esterases to the following primary metabolites: para-aminobenzoic acid and diethylaminoethanol. The activity of both metabolites is unspecified. Biological Half-Life Tetracaine is hydrolyzed rapidly in the plasma; therefore, half-life has not been determined. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the use of tetracaine during breastfeeding. Based on the low excretion of other local anesthetics into breastmilk, a single dose of injected tetracaine during breastfeeding, such as for a dental procedure, is unlikely to adversely affect the breastfed infant. However, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. Topical application of tetracaine to the mother is unlikely to affect her breastfed infant if it is applied away from the breast. Only water-miscible cream or gel products should be applied to the breast because ointments may expose the infant to high levels of mineral paraffins via licking.[1] ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Tetracaine is rapidly hydrolyzed in the plasma; therefore, protein binding could not be determined. |
| 其他信息 |
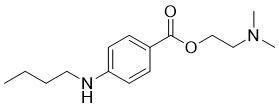
Tetracaine is a benzoate ester in which 4-N-butylbenzoic acid and 2-(dimethylamino)ethanol have combined to form the ester bond; a local ester anaesthetic (ester caine) used for surface and spinal anaesthesia. It has a role as a local anaesthetic. It is a benzoate ester and a tertiary amino compound.
Tetracaine is an ester local anaesthetic currently available in combination with lidocaine as a cream and patch. Tetracaine is an Ester Local Anesthetic. The physiologic effect of tetracaine is by means of Local Anesthesia. Tetracaine is a benzoate ester with anesthetic properties. Upon administration, tetracaine reversibly binds voltage-gated sodium ion channels in neuronal cell membranes and inhibits sodium influx. This prevents the initiation and conduction of nerve impulses, and stabilizes neuronal membranes. This results in a loss of sensation, and thereby provides analgesia and anesthesia. A potent local anesthetic of the ester type used for surface and spinal anesthesia. See also: Tetracaine Hydrochloride (has salt form); Lidocaine; Tetracaine (component of); Benzocaine; Lidocaine; Tetracaine (component of) ... View More ... Drug Indication Ophthalmic tetracaine is indicated for the for procedures requiring a rapid and short- acting topical ophthalmic anesthetic. The combination lidocaine and tetracaine patch is indicated for local dermal analgesia for superficial dermatological procedures and superficial venous access. The combination lidocaine and tetracaine cream is intended to provide topical local analgesia for superficial dermatological procedures. FDA Label Mechanism of Action Tetracaine is an ester-type anesthetic and produces local anesthesia by blocking the sodium ion channels involved in the initiation and conduction of neuronal impulses. |
| 精确质量 |
264.183
|
|---|---|
| CAS号 |
94-24-6
|
| 相关CAS号 |
Tetracaine hydrochloride;136-47-0
|
| PubChem CID |
5411
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.0±0.1 g/cm3
|
| 沸点 |
389.4±27.0 °C at 760 mmHg
|
| 熔点 |
43 °C
|
| 闪点 |
189.3±23.7 °C
|
| 蒸汽压 |
0.0±0.9 mmHg at 25°C
|
| 折射率 |
1.538
|
| LogP |
3.65
|
| tPSA |
41.57
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
249
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1C=CC(NCCCC)=CC=1)OCCN(C)C
|
| InChi Key |
GKCBAIGFKIBETG-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H24N2O2/c1-4-5-10-16-14-8-6-13(7-9-14)15(18)19-12-11-17(2)3/h6-9,16H,4-5,10-12H2,1-3H3
|
| 化学名 |
2-(dimethylamino)ethyl 4-(butylamino)benzoate
|
| 别名 |
Tetracaine Pontocaine Amethocaine
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 43 mg/mL (~162.66 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.46 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.46 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.46 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT02863679 | COMPLETED | Drug: Tetracaine hydrochloride gel | Hysteroscopy | Wenzhou Medical University | 2016-05 | Not Applicable |
| NCT03749915 | UNKNOWN STATUS | Device: Pain Ease Cold Spray Drug: Ametop |
Analgesia Topical Anesthetic |
University of British Columbia | 2018-11-20 | Not Applicable |
| NCT02750137 | COMPLETED | Drug: Tetracaine | Adverse Drug Event | KK Women's and Children's Hospital | 2014-08 | |
| NCT02771392 | UNKNOWN STATUS | Drug: Ophthalmic Tetracaine Other: Normal Saline |
Corneal Abrasion | New York Presbyterian Brooklyn Methodist Hospital | 2016-06 | Phase 2 Phase 3 |
| NCT01864213 | COMPLETED | Drug: Ametop cream | Pain | University of British Columbia | 2013-05 | Phase 1 |