| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
50 和 100 μM 时,三氯生(1-100 μM;24 小时)会以剂量和时间依赖性方式降低细胞活力。 50 μM 剂量的三氯生可显着提高蛋白质 Bax 和裂解的 caspase 3,同时降低 Bcl-2[2]。当暴露于 50 μM 三氯生 1-3 小时时,磷酸化 p38 和 JNK 蛋白的表达会更高 [2]。在培养的 NSC 中,50 μM 的三氯生(10–50 μM;3 小时)会降低 GSH 活性,并将 ROS 生成量提高至 40% 左右 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
三氯生(5、50 和 500 mg/kg;口服灌胃,每周 5 天,持续 4 周)诱导产生 IL-4、IL-13 和抗 Der f IgE [3]。
|
| 细胞实验 |
细胞活力测定[2]
细胞类型:神经干孔 测试浓度:1、10、20、30、50 和 100 μM 孵育时间: 24 小时 实验结果: 50 和 100 μM 时,细胞活力以剂量和时间依赖性方式开始降低。 蛋白质印迹分析[2] 细胞类型: 神经干孔 测试浓度: 50 μM 孵育持续时间:1、3小时 实验结果:不影响MAPK信号蛋白本身的表达。差异诱导磷酸化 p38 和 JNK 蛋白表达增加。 |
| 动物实验 |
Animal/Disease Models: Wild type BALB/cJ mice[3]
Doses: 5, 50, 500 mg/kg Route of Administration: po (oral gavage), five days a week for a total of four weeks Experimental Results: Caused an increase in the production of anti-dermatophagoides farinae (Der f) IgE, IL‐4, and IL‐13, and this resulted in the aggravation of airway hyperresponsiveness in aeroallergen‐exposed wild type mice. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A study conducted in 2000 demonstrated that low amounts of triclosan can be absorbed through skin and can enter the bloodstream. Triclosan is rapidly absorbed and distributed in the human body. Maximum concentrations are reached within three hours after oral intake. However, the metabolism and excretion of the compound is fast. In one study, after in vivo topical application of a 64.5mM alcoholic solution of [(3)H]triclosan to rat skin, 12% radioactivity was recovered in the faeces, 8% in the carcass 1% in the urine, 30% in the stratum corneum and 26% was rinsed from the skin surface at 24 hours after application. The number of personal hygiene products containing triclosan has increased rapidly during the last decade, and triclosan is one of the most common antibacterial compounds used in dentifrices today. However, the extent of triclosan exposure has not yet been well described. The potential risks of generating triclosan-resistant pathogenic microorganisms or of the selection of resistant strains are some areas of concern. The aim of the present study was to (1) obtain information on baseline levels of triclosan in plasma and urine, and (2) study the pharmacokinetic pattern of triclosan after a single-dose intake. Ten healthy volunteers were exposed to a single oral dose of 4 mg triclosan by swallowing an oral mouthwash solution. Triclosan in plasma and urine was followed before and up to 8 days after exposure. Triclosan levels in plasma increased rapidly, with a maximum concentration within 1 to 3 hr, and the terminal plasma half-life was 21 hr. The major fraction was excreted within the first 24 hr. The accumulated urinary excretion varied between the subjects, with 24 to 83% of the oral dose being excreted during the first 4 d after exposure. In conclusion, triclosan appears to be readily absorbed from the gastrointestinal tract and has a rapid turnover in humans. The high lipid solubility of the substance gives rise to questions regarding distribution properties and accumulation. The findings of the present study form a basis for greater understanding of the toxicokinetic properties of triclosan in humans. /MILK/ Triclosan amounts in breast milk were reported to range from <20 to 300 ug/kg lipid in one study and <5 to 2100 ug/kg lipid in another. In a study that compared triclosan levels in women who used triclosan-containing products with those who did not, levels in breast milk were 0.022 to 0.95 ug/kg lipid compared to 0.018 to 0.35 ug/kg lipid, respectively. Oral and dermal routes (humans and rodents): Triclosan glucuronide is predominantly excreted in the urine, and triclosan is predominantly excreted in the feces. Triclosan that is administered orally and dermally is excreted in greater concentrations in the urine than in the feces in humans, hamsters, rabbits, and monkey. In rats, mice, and dog, the reverse is true. Up to 87% of triclosan that is administered to humans (by an unspecified route) is excreted in the urine, most of it within 72 hr after dose. The percutaneous absorption of unlabeled triclosan was investigated in a pilot study and a 90-day study with infant Rhesus monkeys. In the pilot study, triclosan was detected in all blood samples following a single dermal exposure to a soap solution containing triclosan (1 mg/mL, 0.1%), with blood levels detected up to 24 hours, and peak levels observed at 8 to 12 hours. In the 90-day study, only the glucuronide and sulfate conjugates were detected in blood samples, the glucuronide predominating in the early blood samples (Days 1 to 2), and triclosan sulfate predominating in all subsequent blood samples (samples were taken daily for the 90-day duration of the study). Triclosan was excreted in the urine primarily as the glucuronide conjugate, but was excreted in the feces primarily in the free or unconjugated form. Low levels of triclosan were detected in tissues. The results of this monkey study indicate that triclosan was absorbed percutaneously following 90 days of daily washing with 15 mL of soap (1 mg triclosan/mL) and that the proportion of plasma glucuronide and sulfate conjugates altered following chronic administration. For more Absorption, Distribution and Excretion (Complete) data for Triclosan (14 total), please visit the HSDB record page. Metabolism / Metabolites Triclosan is prone to phase II metabolism via sulfotransferase and glucuronosyltransferase enzymes (Wang et al., 2004). In humans the resulting conjugates are excreted primarily in urine. Oral and dermal routes (humans and rodents): Triclosan absorbed from the gastrointestinal tract undergoes extensive first-pass metabolism, which primarily involves glucuronide and sulfate conjugation. In both humans and rodents, at high triclosan plasma concentrations, metabolism shifts from the generation of predominantly glucuronide conjugates to sulfate-conjugates. The bioavailability of unconjugated triclosan may be limited after oral exposure because of triclosan's extensive first-pass metabolism. Triclosan is also metabolized to its glucuronide and sulfate conjugates by the skin. Triclosan has been widely used as a disinfectant in human health care products. Although this particular chemical is less toxic, its biotransformation products might have toxicity to human. Therefore, understanding the pharmacokinetics and metabolism of triclosan in animal and human body is important. Plasma samples from SD rats collected after the oral administration of 5 mg/kg triclosan were analyzed ... . The pharmacokinetic data of triclosan in the rats were presented including the half time of elimination that was (48.5 +/- 10.5) hr, indicating that the elimination of triclosan in the rat was slow. Two hydroxylated and sulfonated triclosan, one glucuronidated triclosan, and one sulfonated triclosan were identified in the rat plasma samples. ...Irgasan DP 300 is excreted unchanged in feces and urine (partly conjugated) but is also hydroxylated to five different monohydroxy metabolites which were found in urine; three of these were also present in feces. Triclosan has known human metabolites that include Triclosan sulfate and (2S,3S,4S,5R)-6-[5-chloro-2-(2,4-dichlorophenoxy)phenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid. Triclosan is prone to phase II metabolism via sulfotransferase and glucuronosyltransferase enzymes (Wang et al., 2004). In humans the resulting conjugates are excreted primarily in urine (Sandborgh-Englund et al., 2006). In one study, after in vivo topical application of a 64.5mM alcoholic solution of [(3)H]triclosan to rat skin, 12% radioactivity was recovered in the faeces, 8% in the carcass 1% in the urine, 30% in the stratum corneum and 26% was rinsed from the skin surface at 24 hours after application. (A7866) The terminal plasma half life of triclosan is 21 h (Sandborgh-Englund et al., 2006). Biological Half-Life The terminal plasma half life of triclosan is 21 h. Measurements of the oral retention and pharmacokinetics of (3)H-triclosan delivered from a toothpaste containing 0.2% (3)H-triclosan were made in 12 human volunteers (aged 19-37 yr). ... After use of a one g quantity of the toothpaste, 36.3+-1.4% of the (3)H-triclosan was retained. ... The saliva decay curve for (3)H-triclosan was consistent with a 2 phase model with half life values of 0.45 hr and 2.42 hr, respectively. ... ... Ten healthy volunteers were exposed to a single oral dose of 4 mg triclosan by swallowing an oral mouthwash solution. Triclosan in plasma and urine was followed before and up to 8 days after exposure. Triclosan levels in plasma increased rapidly, with a maximum concentration within 1 to 3 hr, and the terminal plasma half-life was 21 hr. ... The blood half-life of (14)C-triclosan during the beta-phase was 8.8 +/- 0.6 hr and the blood clearance rate was 77.5 +/- 11.3 mL/kg/hr /after injection either via the femoral vein, (iv, 5 mg/kg in polyethylene glycol-400) or into the vaginal orifice (ivg, 5 mg/kg in corn oil)/ . Plasma samples from SD rats collected after the oral administration of 5 mg/kg triclosan were analyzed ... . The pharmacokinetic data of triclosan in the rats were presented including the half time of elimination that was (48.5 +/- 10.5) hr, indicating that the elimination of triclosan in the rat was slow. Two hydroxylated and sulfonated triclosan, one glucuronidated triclosan, and one sulfonated triclosan were identified in the rat plasma samples. |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Triclosan is a white to off-white crystalline powder. It is used as fungicide and bacteriostat. It is highly active against staphylococci and is used as an active agent in deodorants and antiseptic soaps. It is also used as material preservative for industrial and household plastics and textiles. HUMAN STUDIES: Fifty human subjects were treated with a 0.5% triclosan solution in a 1% soap solution. Triclosan was found not to be a sensitizer and the irritation potential depended on concentration. Tests also showed that triclosan was not a photosensitizing agent. Triclosan exposure might cause spontaneous abortion; probably through inhibition of estrogen sulfotransferase activity to produce placental thrombosis. In terms of estrogenic activity, triclosan displaced (3)H-estradiol from estrogen receptors (ER) of MCF7 human breast cancer cells and from recombinant human ER alpha/ER beta. In children, triclosan exposure was associated with allergic sensitization, especially inhalant and seasonal allergens, rather than food allergens. Current rhinitis was associated with the highest levels of triclosan, whereas no association was seen for current asthma. ANIMAL STUDIES: Triclosan was found to cause slight primary eye irritation when applied to the rabbit eye mucosa. Triclosan had a very low sensitization index in guinea pigs. Mice and rats administered triclosan intravenously at 10, 20 and 30 (rats only) mg/kg bw showed signs of slight cramps, exopthalmos (mice only), mydriasis (rats only), dyspnea, and ventral decubitus, with recovery by 24 to 48 hr after dosing. In an 18-month carcinogenicity bioassay in mice, 5 groups of male and female mice (70 mice/sex/dose) received triclosan in the diet at dose levels of 0, 10, 30, 100, or 200 mg/kg/day. Fifty mice/sex/dose received dietary triclosan for 18 months, while the remaining 20 mice/sex/dose received dietary triclosan for only 6 months, after which time these mice were sacrificed. After 18 months of exposure, a statistically significant increase in the incidence of hepatocellular adenoma and/or carcinoma was observed in male and female mice at 100 mg/kg/day triclosan and above. The incidence was dose-related in both sexes. In a developmental toxicity study in mice, triclosan was administered to 25 female mice/dose via the diet at target dose levels of 0, 10, 25, 75, or 350 mg/kg/day from days 6-15 of gestation. Maternal toxicity was observed at 75 mg/kg/day by increases in absolute and relative liver weight and tan areas in the liver of one dam. Developmental effects were noted at the 75 and 350 mg/kg/day target dose levels as increased incidence of variations (characterized as reversible irregular ossification of the skull at 75 and 350 mg/kg/day, and phalanges at 350 mg/kg/day). Decreased fetal weight was also observed 14 and 18% decrease at 75 and 350 mg/kg/day target dose levels, respectively). Triclosan decreased the synthesis of androgens followed by reduced sperm production in treated male rats. In rats, direct per oral pup exposure from postnatal day 3-16 to 50 or 150 mg triclosan/kg bw/day was performed. This exposure pointed to significant T4 reductions in 16 day old offspring in both dose groups. Triclosan was tested on Salmonella typhimurium strains TA1537, TA100, TA98, TA1535 and TA1538 with or without metabolic activation. There were no treatment-related increases in gene mutation at any dose. Dermal exposure to triclosan induces stimulation of the immune system in a murine model. ECOTOXICITY STUDIES: At environmentally relevant concentrations (<2 ug/L) it could cause a decline in the fish population. Triclosan in the aquatic environment may affect algal growth, chlorophyll synthesis, oxidative stress responses and cause biochemical alterations. Mature male western mosquitofish, Gambusia affinis, were exposed to triclosan concentrations of 100, 200, and 350 nM for 35 days. Vitellogenin mRNA expression was significantly elevated in the 350 nM triclosan treatment. Sperm counts in the same treatment group were significantly decreased. The mean hepatosomatic index in the 350 nM treatment group was significantly increased. This study demonstrates that triclosan has the potential to act as an endocrine disruptor in male mosquitofish. In the North American bullfrog, Rana catesbeiana, exposure to low levels of triclosan disrupts thyroid hormone-associated gene expression and can alter the rate of thyroid hormone-mediated postembryonic anuran development. Triclosan induced systemic toxic effects in C. elegans. At in-use concentrations, triclosan acts as a biocide, with multiple cytoplasmic and membrane targets. At lower concentrations, however, triclosan appears bacteriostatic and is seen to target bacteria mainly by inhibiting fatty acid synthesis. Triclosan binds to bacterial enoyl-acyl carrier protein reductase enzyme (ENR), which is encoded by the gene FabI. This binding increases the enzyme's affinity for nicotinamide adenine dinucleotide (NAD+). This results in the formation of a stable ternary complex of ENR-NAD+-triclosan, which is unable to participate in fatty acid synthesis. Fatty acids are necessary for reproducing and building cell membranes. Humans do not have an ENR enzyme, and thus are not affected. Toxicity Data Oral LD50, Rat: 3700 mg/kg; Dermal LD50, Rabbit: 9300 mg/kg Interactions Humans are commonly exposed to multiple environmental chemicals, including tetrabromobisphenol A (TBBPA; a flame retardant), triclosan (an antimicrobial agent), and bisphenol A (BPA; polycarbonate plastics). These chemicals are readily absorbed and may interact with each other. We sought to determine whether TBBPA, given alone or in combination with triclosan, can modulate the concentrations of BPA and 17bta-estradiol (E2). Female and male CF-1 mice were each given a subcutaneous injection of 0-27 mg TBBPA, with or without concurrent 0.33 mg triclosan, followed by dietary administration of 50 ug/kg body weight (14)C-BPA. Radioactivity was measured in blood serum and tissues through liquid scintillation counting. In subsequent experiments, female and male CF-1 mice were each given a subcutaneous injection of 0 or 1 mg TBBPA and E2 was measured in urine 2-12 hr after injection. Doses as low as 1mg TBBPA significantly elevated (14)C-BPA concentrations in the uterus and ovaries of females; in the testes, epididymides, vesicular-coagulating glands, and preputial glands of males; and in blood serum, heart, lungs, and kidneys of both sexes; urinary E2 concentrations were also elevated. Lower doses of TBBPA or triclosan that had no effects on their own elevated (14)C-BPA concentrations when the two substances were given concurrently. These data indicate that TBBPA, triclosan, and BPA interact in vivo, consistent with evidence that TBBPA and triclosan inhibit enzymes that are critical for BPA and E2 metabolism. Atrazine is an herbicide with several known toxicologically relevant effects, including interactions with other chemicals. Atrazine increases the toxicity of several organophosphates and has been shown to reduce the toxicity of triclosan to Daphnia magna in a concentration dependent manner. Atrazine is a potent activator in vitro of the xenobiotic-sensing nuclear receptor, HR96, related to vertebrate constitutive androstane receptor (CAR) and pregnane X-receptor (PXR). RNA sequencing (RNAseq) was performed to determine if atrazine is inducing phase I-III detoxification enzymes in vivo, and estimate its potential for mixture interactions. RNAseq analysis demonstrates induction of glutathione S-transferases (GSTs), cytochrome P450s (CYPs), glucosyltransferases (UDPGTs), and xenobiotic transporters, of which several are verified by qPCR. Pathway analysis demonstrates changes in drug, glutathione, and sphingolipid metabolism, indicative of HR96 activation. Based on our RNAseq data, we hypothesized as to which environmentally relevant chemicals may show altered toxicity with co-exposure to atrazine. Acute toxicity tests were performed to determine individual LC50 and Hillslope values as were toxicity tests with binary mixtures containing atrazine. The observed mixture toxicity was compared with modeled mixture toxicity using the Computational Approach to the Toxicity Assessment of Mixtures (CATAM) to assess whether atrazine is exerting antagonism, additivity, or synergistic toxicity in accordance with our hypothesis. Atrazine-triclosan mixtures showed decreased toxicity as expected; atrazine-parathion, atrazine-endosulfan, and to a lesser extent atrazine-p-nonylphenol mixtures showed increased toxicity. In summary, exposure to atrazine activates HR96, and induces phase I-III detoxification genes that are likely responsible for mixture interactions. ... The aim of the present study was to examine whether triclosan reduces the clinical symptoms on skin after exposure to nickel in an allergic patch test reaction (APR) model. 1% nickel sulfate was used for APR in 10 nickel-allergic females. The results showed that application of triclosan on skin reduced the APR symptoms from nickel in sensitized patients significantly (p<0.05) compared to the saline and alcohol solutions. ... The aim of the present study was to examine whether triclosan has an effect on the inflammation in human skin caused by intradermal administration of histamine. 9 female volunteers participated in a double-blind study, and skin patch tests were performed in 2 series. In the 1st, the skin was pre-treated for 1 hr with triclosan before the histamine was applied. In the 2nd, the histamine reaction was elicited first and triclosan applied subsequently. The effect of triclosan on the wheals formed in the skin after histamine application was measured. It was found that triclosan reduced the size of the wheals markedly when triclosan was applied after the wheals were formed, and that pre-treatment of the skin had only a slight effect. ... The effects of triclosan /were evaulated/ in the female Wistar rat following exposure for 21 days in the Endocrine Disruptor Screening Program pubertal protocol and the weanling uterotrophic assay (3-day exposure). In the pubertal study, triclosan advanced the age of onset of vaginal opening and increased uterine weight at 150 mg/kg, indicative of an estrogenic effect. In the uterotrophic assay, rats received oral doses of triclosan (1.18, 2.35, 4.69, 9.37, 18.75, 37.5, 75, 150, and 300 mg/kg) alone, 3 ug/kg ethinyl estradiol (EE), or triclosan (same doses as above) plus 3 ug/kg EE. Uterine weight was increased in the EE group (positive control) as compared with the control but was not affected by triclosan alone. However, there was a significant dose-dependent increase in the group cotreated with EE and triclosan (> or = 4.69 mg/kg) as compared with EE alone, indicating a potentiation of the estrogen response on uterine weight. This result was well correlated with potentiated estrogen-induced changes in uterine histology. Serum thyroid hormone concentrations were also suppressed by triclosan in this study, similar to other studies in the male and female rat. In conclusion, triclosan affected estrogen-mediated responses in the pubertal and weanling female rat and also suppressed thyroid hormone in both studies. The lowest effective concentrations in the rodent model are approximately 10 (for estrogen) and 40 (for thyroid hormone) times higher than the highest concentrations reported in human plasma. Non-Human Toxicity Values LD50 Rat iv 19 mg/kg LD50 Rat sc 3900 mg/kg LD50 Rat dermal 9300 mg/kg LD50 Rat oral 3700 mg/kg For more Non-Human Toxicity Values (Complete) data for Triclosan (7 total), please visit the HSDB record page. |
| 参考文献 |
[1]. Weatherly LM, et al. Triclosan exposure, transformation, and human health effects. J Toxicol Environ Health B Crit Rev. 2017;20(8):447-469.
[2]. Ley C, et al. Triclosan and triclocarban exposure and thyroid function during pregnancy-A randomized intervention. Reprod Toxicol. 2017 Dec;74:143-149. |
| 其他信息 |
Therapeutic Uses
Anti-Infective Agents, Local; Fatty Acid Synthesis Inhibitors /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Triclosan is included in the database. Control of meticillin-resistant Staphylococcus aureus (MRSA) infection in surgical units has been achieved by procedures including handwashing and bathing with triclosan. Triclosan is a chlorinated bisphenol antiseptic, effective against Gram-positive and most Gram-negative bacteria but with variable or poor activity against Pseudomonas spp. It is also active against fungi. It is used in soaps, creams, and solutions in concentration of up to 2% for disinfection of the hands and wounds and for disinfection of the skin prior to surgery, injections, or venepuncture. It is also used in oral hygiene products and in preparations for acne. For more Therapeutic Uses (Complete) data for Triclosan (7 total), please visit the HSDB record page. |
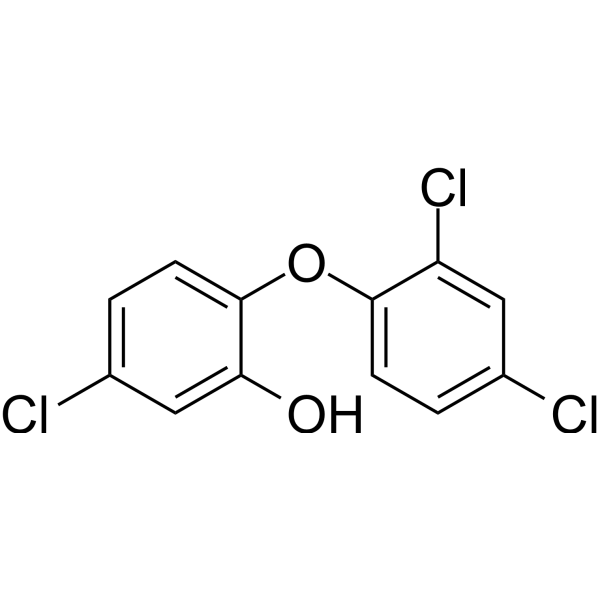
| 分子式 |
C12H7CL3O2
|
|---|---|
| 分子量 |
289.54178071022
|
| 精确质量 |
287.951
|
| CAS号 |
3380-34-5
|
| 相关CAS号 |
Triclosan-d3;1020719-98-5
|
| PubChem CID |
5564
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
344.6±42.0 °C at 760 mmHg
|
| 熔点 |
56-60 °C(lit.)
|
| 闪点 |
162.2±27.9 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.632
|
| LogP |
5.17
|
| tPSA |
29.46
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
17
|
| 分子复杂度/Complexity |
252
|
| 定义原子立体中心数目 |
0
|
| InChi Key |
XEFQLINVKFYRCS-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H
|
| 化学名 |
5-chloro-2-(2,4-dichlorophenoxy)phenol
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 100 mg/mL (~345.38 mM)
H2O : ~1 mg/mL (~3.45 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (8.63 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (8.63 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.4538 mL | 17.2688 mL | 34.5375 mL | |
| 5 mM | 0.6908 mL | 3.4538 mL | 6.9075 mL | |
| 10 mM | 0.3454 mL | 1.7269 mL | 3.4538 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。