| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
MEK2 (IC50 = 60 nM); MEK1 (IC50 = 70 nM)
Mitogen-activated protein kinase kinase 1 (MEK1) and MEK2, serine/threonine kinases in the MAPK pathway. For U0126-EtOH, the IC50 values were: MEK1 = 70 nM, MEK2 = 60 nM (radioactive kinase assay) [1] - Consistent with [1], MEK1 (IC50 = 80 nM) and MEK2 (IC50 = 75 nM) via fluorogenic peptide assay; no inhibition of other kinases (e.g., ERK1, JNK, p38, PI3K) at 10 μM, confirming MEK1/2 selectivity [6] |
|---|---|
| 体外研究 (In Vitro) |
U0126-EtOH 通过功能性拮抗 AP-1 转录活性来阻断参与炎症反应的多种细胞因子和金属蛋白酶的产生。 [1]通过降低 IL-2 mRNA 水平,U0126-EtOH 可减少响应抗原刺激或交联抗 CD3 加抗 CD28 抗体的 T 细胞增殖,而不影响 IL-2 引起的增殖。 [2]根据最近的一项研究,U0126-EtOH 抑制线粒体功能,在不借助 MEK 的情况下将去势抵抗性人类前列腺癌 C4-2 细胞转变为有氧糖酵解,并抵消白藜芦醇诱导的细胞凋亡。 [3]
酶活与细胞MAPK抑制:U0126-EtOH(0.01 μM–10 μM)可剂量依赖性抑制重组MEK1/2,并降低HeLa细胞中p-ERK水平:1 μM处理2小时后p-ERK减少50%(Western blot) [1]。在COS-7细胞中,5 μM处理1小时可阻断EGF诱导的p-ERK(减少90%) [6] - T细胞功能调控:在抗CD3/CD28抗体刺激的人外周血T细胞中,U0126-EtOH(1 μM–20 μM)抑制IL-2分泌(10 μM时减少60%,ELISA)和T细胞增殖(MTT法72小时IC50=8 μM),且无显著T细胞死亡(20 μM时细胞毒性<10%) [2] - 癌细胞增殖抑制:在KRAS突变结直肠癌细胞(HT-29)和BRAF突变黑色素瘤细胞(A375)中,U0126-EtOH(0.5 μM–30 μM)抑制增殖:MTT法72小时IC50分别为HT-29 5 μM、A375 3 μM。Western blot显示5 μM时p-ERK减少80%、cyclin D1减少55% [3] - 抗病毒活性:在感染登革病毒(DENV-2)的Vero细胞中,U0126-EtOH(1 μM–15 μM)减少病毒复制:qRT-PCR检测48小时病毒RNA的EC50=4 μM,5 μM时病毒蛋白NS3减少70%(Western blot),且无细胞毒性(15 μM时存活率>85%) [4] |
| 体内研究 (In Vivo) |
U0126-EtOH 通过抑制细胞内 Raf/MEK/ERK 信号通路,抑制 2009 年大流行 IV H1N1 变种和高致病性禽流感病毒 (HPAIV) 在小鼠肺体内的传播,从而表现出抗病毒活性。 [4]通过在注射 A 的大鼠中激活核呼吸因子 1、线粒体转录因子 A 和过氧化物酶体增殖物激活受体 γ 共激活剂-1a,U0126-EtOH 表现出潜在的神经保护作用并改善莫里斯水迷宫 (MWM) 中的空间学习。 5]
结直肠癌异种移植模型:6周龄雌性裸鼠接种HT-29细胞,随机分为2组(每组n=6):溶媒组(DMSO+生理盐水)、U0126-EtOH 20 mg/kg组。药物腹腔注射每日一次,连续14天。肿瘤体积较溶媒组减少45%,肿瘤重量减少40%。免疫组化显示p-ERK减少65%、Ki-67减少50% [3] - 登革病毒小鼠模型:4周龄雌性BALB/c小鼠感染DENV-2后,用U0126-EtOH(15 mg/kg,腹腔注射每日一次)处理7天。血清病毒载量较溶媒组减少60%(qRT-PCR),小鼠存活率从40%(溶媒组)升至75% [4] - T细胞介导免疫模型:雄性C57BL/6小鼠用卵清蛋白免疫后,用U0126-EtOH(10 mg/kg,口服每日一次)处理10天。脾T细胞IL-2分泌较溶媒组减少50%(ELISA),证实体内MEK抑制活性 [2] |
| 酶活实验 |
在这些测定中,调整免疫沉淀的野生型 MEK 的量以产生与 10 nM 重组 MEK 相同数量的活性单位。 96 孔硝化纤维过滤器装置用于测量反应速度,如下详述。除非另有说明,所有反应均使用 10 nM 酶浓度、20 mM Hepes、10 mM MgCl2、5 mM β-巯基乙醇、0.1 mg/mL BSA、pH 7.4 和室温。向预混合的 MEK/ERK/抑制剂反应混合物中添加 [γ-33P]ATP 以启动反应。然后每 6 分钟取出 100 μL 等分试样,转移至含有 50 mM EDTA 的 96 孔硝酸纤维素膜板中以终止反应。将膜板拉出并用缓冲液真空清洗四次。然后将30μL Microscint-20闪烁液倒入孔中,使用Top Count闪烁计数器测量33P-磷酸化ERK的放射性。根据放射性与时间图的斜率,可以计算出速度。除非另有说明,ERK 和 ATP 浓度分别为 400 nM 和 40 μM。存在和不存在抑制剂时的初始反应速度分别称为 Vi 和 Vo,它们用于计算所有体外酶测定的抑制百分比。然后将数据绘制为抑制剂浓度与抑制百分比的函数关系,并通过使用非线性最小二乘回归将数据拟合到 Langmuir 等温线方程来计算 IC50。根据提供的信息,酶浓度不是基于活性位点滴定,而是基于最终测定体积中使用的蛋白质的分子量和质量。因此,酶活性位点的实际浓度可能与报告值不同。
MEK1/2放射性激酶实验:将重组人MEK1(3–321位氨基酸)或MEK2(4–317位氨基酸)与[γ-³²P]-ATP(10 μM,3000 Ci/mmol)、重组ERK2(底物激酶)及髓鞘碱性蛋白(MBP,底物)共同孵育于实验缓冲液(25 mM Tris-HCl pH 7.5、10 mM MgCl₂、1 mM DTT、0.1 mM Na₃VO₄)中。加入系列稀释的U0126-EtOH(0.01 μM–10 μM),30°C孵育30分钟。加入30%三氯乙酸(TCA)终止反应,将沉淀的MBP转移至P81磷酸纤维素滤膜。用1%磷酸洗涤滤膜3次,液体闪烁计数仪检测放射性,四参数逻辑回归计算IC50 [1] - MEK荧光实验:将重组MEK1/2与荧光乙酰化肽(Ac-KK(Ac)-AMC,20 μM)及NAD⁺(500 μM)共同孵育于缓冲液(50 mM Tris-HCl pH 8.0、1 mM DTT)中。加入U0126-EtOH(0.01 μM–10 μM),37°C孵育60分钟,检测荧光强度(激发光360 nm,发射光460 nm)以评估MEK抑制效率 [6] |
| 细胞实验 |
A.E7 或 Th17 细胞与已用丝裂霉素 C 以及不同浓度的鸽子细胞色素 c、PR8 Ag 或 5 U/mL 人 rIL-2 处理的 B10.BR 或 BALB/c 脾细胞一起孵育。为了确定 MEK 抑制对 T 细胞增殖的直接影响,一些测定还包含 U0126 或无活性类似物 U0124。每个孔在培养开始两天后接受 1-μCi [3H]TdR 脉冲,并在第二天收获培养物。在不使用液体闪烁混合物的情况下,在 Packard Matrix 96 直接 β 计数器上测量 [3H]TdR 掺入 DNA 的情况。
T细胞增殖与细胞因子实验:分离人外周血T细胞,以2×10⁵个细胞/孔接种于96孔板,加入抗CD3/CD28抗体。加入U0126-EtOH(0.5 μM–20 μM),37°C、5% CO₂孵育72小时。MTT法检测增殖(计算IC50),收集培养上清液ELISA定量IL-2 [2] - 癌细胞Western blot与增殖实验:将HT-29/A375细胞以3×10⁵个细胞/孔接种于6孔板,用U0126-EtOH(0.5 μM–10 μM)处理24小时。RIPA缓冲液裂解细胞,Western blot检测抗p-ERK、抗cyclin D1及抗GAPDH。增殖实验中,细胞接种于96孔板,用U0126-EtOH处理72小时后进行MTT检测 [3] - 抗病毒细胞实验:将Vero细胞以1×10⁵个细胞/孔接种于24孔板,感染DENV-2(MOI=0.1)1小时后加入U0126-EtOH(1 μM–15 μM),孵育48小时。提取总RNA qRT-PCR检测病毒RNA,Western blot检测病毒NS3蛋白 [4] |
| 动物实验 |
Female C57Bl/6 mice infected by Mouse-adapted highly pathogenic avian influenza A/FPV/Bratislava/79 (H7N7; FPV) virus and swine origin human influenza A virus (SOIV) A/Regensburg/D6/2009 (H1N1v; RB1).
≤10 mM Administered via aerosol. |
| 毒性/毒理 (Toxicokinetics/TK) |
In Vitro Cytotoxicity: In normal human foreskin fibroblasts (NHFF) and Vero cells, U0126-EtOH (up to 20 μM, 72 h) showed viability > 80%, indicating low non-specific cytotoxicity [3][4]
- In Vivo Acute Toxicity: In BALB/c mice treated with U0126-EtOH (up to 50 mg/kg, intraperitoneal, 7 days), no significant weight loss, lethargy, or organ damage (liver/kidney histology) was observed [4] |
| 参考文献 | |
| 其他信息 |
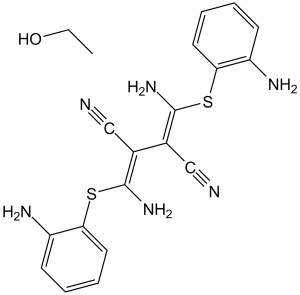
U0126.EtOH is an addition compound obtained by combining equimolar amounts of (2Z,3Z)-bis{amino[(2-aminophenyl)sulfanyl]methylidene}butanedinitrile (U0126) and ethanol. An inhibitor of mitogen-activated protein kinase that also exhibits anti-cancer properties. It has a role as an EC 2.7.11.24 (mitogen-activated protein kinase) inhibitor, an apoptosis inducer, an antineoplastic agent, an antioxidant, an osteogenesis regulator and a vasoconstrictor agent. It contains an U0126.
\n\nIn search of antiinflammatory drugs with a new mechanism of action, U0126 was found to functionally antagonize AP-1 transcriptional activity via noncompetitive inhibition of the dual specificity kinase MEK with an IC50 of 0.07 microM for MEK 1 and 0.06 microM for MEK 2. U0126 can undergo isomerization and cyclization reactions to form a variety of products, both chemically and in vivo, all of which exhibit less affinity for MEK and lower inhibition of AP-1 activity than parent, U0126.[1] \n\nThree mitogen-activated protein kinase pathways are up-regulated during the activation of T lymphocytes, the extracellular signal-regulated kinase (ERK), Jun NH2-terminal kinase, and p38 mitogen-activated protein kinase pathways. To examine the effects of blocking the ERK pathway on T cell activation, we used the inhibitor U0126, which has been shown to specifically block mitogen-activated protein kinase/ERK kinase (MEK), the kinase upstream of ERK. This compound inhibited T cell proliferation in response to antigenic stimulation or cross-linked anti-CD3 plus anti-CD28 Abs, but had no effect on IL-2-induced proliferation. The block in T cell proliferation was mediated by down-regulating IL-2 mRNA levels. Blocking Ag-induced proliferation by inhibiting MEK did not induce anergy, unlike treatments that block entry into the cell cycle following antigenic stimulation. Surprisingly, induction of anergy in T cells exposed to TCR cross-linking in the absence of costimulation was also not affected by blocking MEK, unlike cyclosporin A treatment that blocks anergy induction. These results suggest that inhibition of MEK prevents T cell proliferation in the short term, but does not cause any long-term effects on either T cell activation or induction of anergy. These findings may help determine the viability of using mitogen-activated protein kinase inhibitors as immune suppressants.[2] \n\nRecent evidence has identified substantial overlap between metabolic and oncogenic biochemical pathways, suggesting novel approaches to cancer intervention. For example, cholesterol lowering statins and the antidiabetes medication metformin both act as chemopreventive agents in prostate and other cancers. The natural compound resveratrol has similar properties: increasing insulin sensitivity, suppressing adipogenesis, and inducing apoptotic death of cancer cells in vitro. However, in vivo tumor xenografts acquire resistance to resveratrol by an unknown mechanism, while mouse models of metabolic disorders respond more consistently to the compound. Here we demonstrate that castration-resistant human prostate cancer C4-2 cells are more sensitive to resveratrol-induced apoptosis than isogenic androgen-dependent LNCaP cells. The MEK inhibitor U0126 antagonized resveratrol-induced apoptosis in C4-2 cells, but this effect was not seen with other MEK inhibitors. U0126 was found to inhibit mitochondrial function and shift cells to aerobic glycolysis independently of MEK. Mitochondrial activity of U0126 arose through decomposition, producing both mitochondrial fluorescence and cyanide, a known inhibitor of complex IV. Applying U0126 mitochondrial inhibition to C4-2 cell apoptosis, we tested the possibility that glutamine supplementation of citric acid cycle intermediate α-ketoglutarate may be involved. Suppression of the conversion of glutamate to α-ketoglutarate antagonized resveratrol-induced death in C4-2 cells. A similar effect was also seen by reducing extracellular glutamine concentration in the culture medium, suggesting that resveratrol-induced death is dependent on glutamine metabolism, a process frequently dysregulated in cancer. Further work on resveratrol and metabolism in cancer is warranted to ascertain if the glutamine dependence has clinical implications.[3] U0126-EtOH is a widely used small-molecule inhibitor of MEK1/2, primarily employed as a research tool to study MAPK pathway function in cancer, immunology, and virology [1][2][3][4][6] - Its mechanism involves competitive binding to MEK1/2’s ATP-binding pocket, inhibiting MEK-mediated ERK phosphorylation, thereby blocking downstream cell proliferation, cytokine secretion, or viral replication [1][3][4][6] - It is not approved for clinical use and lacks formal ADME/pharmacokinetic data, as it is designed for in vitro and preclinical research rather than therapeutic development [1][3][6] |
| 分子式 |
C18H16N6S2.C2H6O
|
|---|---|
| 分子量 |
426.56
|
| 精确质量 |
426.129
|
| 元素分析 |
C, 56.32; H, 5.20; N, 19.70; O, 3.75; S, 15.03
|
| CAS号 |
1173097-76-1
|
| 相关CAS号 |
U0126;109511-58-2; 218601-62-8 (ZZ-isomer); 218601-64-0 (EE-isomer); 1173097-76-1 (EtOH)
|
| PubChem CID |
16220066
|
| 外观&性状 |
White to off-white solid powder
|
| 沸点 |
612.5ºC at 760 mmHg
|
| 闪点 |
324.2ºC
|
| LogP |
5.684
|
| tPSA |
222.49
|
| 氢键供体(HBD)数目 |
5
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
612
|
| 定义原子立体中心数目 |
0
|
| SMILES |
S(/C(=C(\C#N)/C(/C#N)=C(\N([H])[H])/SC1=C([H])C([H])=C([H])C([H])=C1N([H])[H])/N([H])[H])C1=C([H])C([H])=C([H])C([H])=C1N([H])[H].O([H])C([H])([H])C([H])([H])[H]
|
| InChi Key |
CFQULUVMLGZVAF-OYJDLGDISA-N
|
| InChi Code |
InChI=1S/C18H16N6S2.C2H6O/c19-9-11(17(23)25-15-7-3-1-5-13(15)21)12(10-20)18(24)26-16-8-4-2-6-14(16)22;1-2-3/h1-8H,21-24H2;3H,2H2,1H3/b17-11+,18-12+
|
| 化学名 |
(2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile;ethanol
|
| 别名 |
U0126; U-0126-EtOH; U0126 EtOH; U 0126; U-0126; U0126-EtOH; U 0126-EtOH; U 0126 EtOH; U0126 Ethanol; U0126.EtOH; (2Z,3Z)-2,3-bis(amino(2-aminophenylthio)methylene)succinonitrile compound with ethanol (1:1); (2Z,3Z)-2,3-bis[amino-(2-aminophenyl)sulfanylmethylidene]butanedinitrile;ethanol; 2,3-Bis(amino((2-aminophenyl)thio)methylene)succinonitrile compound with ethanol (1:1); U0126 ethanolate; .U-0126 EtOH
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+50% PEG 300+ddH2O: 28mg/mL 配方 5 中的溶解度: 5 mg/mL (11.72 mM) in 0.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.3443 mL | 11.7217 mL | 23.4434 mL | |
| 5 mM | 0.4689 mL | 2.3443 mL | 4.6887 mL | |
| 10 mM | 0.2344 mL | 1.1722 mL | 2.3443 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
 Increased endothelin type B (ETB) receptor-mediated vasoconstriction and p-ERK1/2 expression in female rat MCAs.J Cereb Blood Flow Metab.2015 Mar;35(3):454-60. |
|---|
 Ischemia-induced increase in smooth muscle endothelin type B (ETB) receptor and p-ERK1/2 expression after transient middle cerebral artery occlusion (tMCAO) is prevented by U0126 treatment. (A) Representative images of ETBreceptor and (B) p-ERK1/2 expression at 48 hours of reperfusion after tMCAO in the occluded and non-occluded middle cerebral artery (MCA) in untreated, vehicle, and U0126 animals. Scale bar: 50 μm. (C) Quantification (mean fluorescence intensity in the smooth muscle cell layer of the occluded MCA normalized to the non-occluded MCA) of ETBreceptor and (D) p-ERK1/2 expression.J Cereb Blood Flow Metab.2015 Mar;35(3):454-60. |
 MEK1/2 inhibition results in improved neurologic function after transient middle cerebral artery occlusion (tMCAO). (A) Infarct volume from untreated (n=7), vehicle (n=4), and U0126-treated (n=9) rats at 48 hours of reperfusion after tMCAO. (B) Representative NeuN immunostaining of brain slices from Bregma −2.2 to +3.8 is shown (1 mm interval). The border between nonlesioned (NeuN positive) and lesioned areas is depicted in the untreated group. (C) Recovery of sensorimotor function up to 14 days after tMCAO with the 28-point neuroscore. (D) Gross neurologic function graded in six levels from 0—no visible defects to 5—death.J Cereb Blood Flow Metab.2015 Mar;35(3):454-60. |
|
|---|
|
Experimental design.J Cereb Blood Flow Metab.2015 Mar;35(3):454-60. |