| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
CGRP receptor ( IC50 = 0.081 nM )
|
|---|---|
| 体外研究 (In Vitro) |
ubrogetant是一种降钙素基因相关肽受体拮抗剂。其作用机制是作为降钙素基因相关肽受体拮抗剂。
Ubrogepant是降钙素基因相关肽(CGRP)受体的小分子抑制剂,可阻断CGRP的作用,CGRP是一种有效的血管扩张剂,被认为在偏头痛中起作用。
|
| 体内研究 (In Vivo) |
默克公司(新泽西州肯尼沃斯)最近发布了一项在群体药代动力学(PK)建模的后期试验中实施干血斑(DBS)的综合策略。我们将这一策略应用于另一个后期临床项目:ubrogepant (MK-1602),一种用于急性治疗偏头痛的新型口服降钙素基因相关肽受体拮抗剂。在实施时,膨润剂正进入第二阶段的发展。实施DBS以获取急性偏头痛事件附近的PK信息,以实现暴露-反应建模。临床终点是一个自发事件,通常发生在门诊就诊之外。因此,该试验的一个创新特征是促进了DBS在门诊环境中的应用。体外和生物分析试验确定了初步方法的可行性和临床进一步评估的适用性。在一期(健康受试者)和二期(目标患者群体)研究中,采用图形化和群体PK方法,同时采集样本,建立了血液和血浆浓度之间的定量关系。这些综合信息已提交给食品和药物管理局进行监管。在获得监管部门批准后,DBS有望用于进一步的临床研究。人群PK模型被用于剖析门诊DBS采集的变异性来源。从该项目中学到的知识为默克公司(Kenilworth, NJ)在临床试验和研究中实施DBS的更广泛的综合战略提供了信息,以提高门诊环境中收集的PK数据的准确性。[1]
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following oral administration, Tmax occurs between 0.7 and 1.5 h. When administered with a high-fat meal, Tmax is delayed by approximately 2 hours and Cmax was reduced by 22% with no significant changes to the AUC. Ubrogepant exhibits dose-proportional pharmacokinetics throughout the entirety of its recommended dosing range. The main route of elimination is fecal/biliary, while renal excretion is comparatively minor - following administration of a single oral dose to healthy subjects, approximately 42% of the dose was recovered unchanged in the feces and 6% was recovered unchanged in the urine. The apparent central volume of distribution following oral administration is approximately 350 L. The apparent oral clearance of ubrogepant is approximately 87 L/h. Metabolism / Metabolites Ubrogepant is eliminated primarily via metabolism, the majority of which is mediated by CYP3A4. Two circulating glucuronide conjugates, along with unchanged parent drug, were found to be the most abundant circulating components in human plasma. The glucuronide metabolites reportedly carry 6000-fold less activity at CGRP receptors and are therefore considered to be pharmacologically inert. Biological Half-Life Ubrogepant has an elimination half-life of 5-7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In preregistration controlled trials of ubrogepant in several thousand patients, mild-to-moderate serum aminotransferase elevations arose in a small percentage of patients (1% to 2%) and overall rates were not different from those in placebo recipients. In the controlled trials and subsequently with general use, there have been no reports of clinically apparent liver injury attributed to ubrogepant. In contrast, telcagepant, the initial oral CGRP receptor antagonist evaluated as therapy for migraine headaches, was abandoned during development because of several instances of clinically apparent liver injury in recipients that was characterized by marked elevations in serum aminotransferase levels and symptoms of fatigue, nausea and abdominal discomfort arising within 2 to 4 weeks of starting therapy which rapidly resolved with prompt stopping of therapy. Similar episodes have not been reported with ubrogepant. Likelihood score: E (unlikely cause of clinically apparent acute liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation There is no published experience with ubrogepant during breastfeeding. Ubrogepant is 87% protein bound, so levels in milk are likely low. If ubrogepant is required by the mother of an older infant, it is not a reason to discontinue breastfeeding, but until more data become available, an alternate drug may be preferred, especially while nursing a newborn or preterm infant. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Ubrogepant is 87% protein-bound _in vitro_, although the specific proteins to which ubrogepant binds have not been elucidated. |
| 参考文献 | |
| 其他信息 |
Pharmacodynamics
Ubrogepant acutely treats migraine headache pain by blocking the activity of a key transmitter involved in migraine pathogenesis. Exposure to ubrogepant can be significantly increased in patients with severe hepatic or renal insufficiency - dose adjustments are required for these patients in order to avoid excessive exposure, and ubrogepant is not recommended in patients with end-stage renal disease. |
| 分子式 |
C29H26F3N5O3
|
|---|---|
| 分子量 |
549.54364
|
| 精确质量 |
549.199
|
| 元素分析 |
C, 63.38; H, 4.77; F, 10.37; N, 12.74; O, 8.73
|
| CAS号 |
1374248-77-7
|
| PubChem CID |
68748835
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.45±0.1 g/cm3(Predicted)
|
| 沸点 |
729.4±60.0 °C(Predicted)
|
| LogP |
4.128
|
| tPSA |
111.27
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
8
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
40
|
| 分子复杂度/Complexity |
1000
|
| 定义原子立体中心数目 |
4
|
| SMILES |
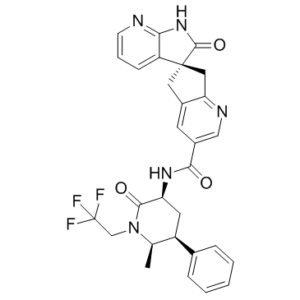
O=C(C1=CN=C2C(C[C@@]3(C4=CC=CN=C4NC3=O)C2)=C1)N[C@@H]5C(N(CC(F)(F)F)[C@H](C)[C@H](C6=CC=CC=C6)C5)=O
|
| InChi Key |
DDOOFTLHJSMHLN-ZQHRPCGSSA-N
|
| InChi Code |
InChI=1S/C29H26F3N5O3/c1-16-20(17-6-3-2-4-7-17)11-22(26(39)37(16)15-29(30,31)32)35-25(38)19-10-18-12-28(13-23(18)34-14-19)21-8-5-9-33-24(21)36-27(28)40/h2-10,14,16,20,22H,11-13,15H2,1H3,(H,35,38)(H,33,36,40)/t16-,20-,22+,28+/m1/s1
|
| 化学名 |
(3S)-N-[(3S,5S,6R)-6-methyl-2-oxo-5-phenyl-1-(2,2,2-trifluoroethyl)piperidin-3-yl]-2-oxospiro[1H-pyrrolo[2,3-b]pyridine-3,6'-5,7-dihydrocyclopenta[b]pyridine]-3'-carboxamide
|
| 别名 |
Ubrogepant; MK1602; MK-1602; 1374248-77-7; Ubrelvy; Ubrogepant anhydrous; UNII-AD0O8X2QJR; AD0O8X2QJR; DTXSID00160178;MK1602; trade name: Ubrelvy
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 100~250 mg/mL (182~454.9 mM)
Ethanol: ~50 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 6.25 mg/mL (11.37 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,将 100 μL 62.5 mg/mL 澄清 DMSO 储备液加入 900 μL 20% SBE-β-CD 生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 6.25 mg/mL (11.37 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 62.5 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 View More
配方 3 中的溶解度: ≥ 5 mg/mL (9.10 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 5%DMSO + 40%PEG300 + 5%Tween 80 + 50%ddH2O: 5.0mg/ml (9.10mM) 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8197 mL | 9.0985 mL | 18.1970 mL | |
| 5 mM | 0.3639 mL | 1.8197 mL | 3.6394 mL | |
| 10 mM | 0.1820 mL | 0.9099 mL | 1.8197 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05125302 | Recruiting | Drug: Ubrogepant Drug: Placebo-Matching Ubrogepant |
Migraine | AbbVie | January 13, 2022 | Phase 3 |
| NCT05214001 | Recruiting | Drug: Almotriptan 12.5 Mg Oral Tablet Drug: Ubrogepant 50Mg Tab |
Migraine With Aura Migraine Without Aura |
Messoud Ashina, MD | June 30, 2022 | Phase 4 |
| NCT05892757 | Recruiting | Drug: Atogepant Drug: Ubrogepant |
Healthy Volunteers | AbbVie | July 11, 2023 | Phase 1 |
| NCT06212661 | Not yet recruiting | Drug: Ubrogepant Drug: Rimegepant Drug: Atogepant |
Migraine Interstitial Cystitis |
The Cleveland Clinic | January 2024 | N/A |
| NCT05827887 | Recruiting | N/A | Migraine | AbbVie | June 25, 2023 | N/A |