| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 体外研究 (In Vitro) |
在平滑肌瘤细胞中,醋酸乌利司他(0.1–5 μM;96 小时)可促进自噬。 Ulipristal 会改变自噬标记物 p62/SQSTM1 和 LC3 的表达。在平滑肌瘤细胞中,ulipristal 上调 Atg7 蛋白 [2]。在培养的子宫肌瘤和平滑肌瘤细胞中,ulipristalacetate 抑制激活素 A 对纤连蛋白和血管内皮生长因子 A (VEGF-A) mRNA 表达的调节 [4]。
|
|---|---|
| 体内研究 (In Vivo) |
在体内,ulipristal 和 CDB-4124 表现出很强的抗妊娠特性 [5]。在所有治疗组中,醋酸乌利司他均降低了腺癌和乳腺纤维腺瘤的发病率。暴露于最高剂量醋酸乌利司他[AUC(0-24h)]的大鼠暴露于人类治疗剂量10mg/天的67倍。当醋酸乌利司他的给药剂量达到治疗暴露量的 313 倍时,小鼠没有表现出任何类型的肿瘤生长。每天服用 130 mg/kg 剂量的雄性和雌性小鼠中,肝脏、垂体、甲状腺/甲状旁腺和附睾重量变化以及最小的整个小叶是与小鼠体内醋酸乌利司他相关的唯一发现。肝细胞增大[6]。与对照相比,醋酸乌利司他(1 mg/kg 和 5 mg/kg)以剂量依赖性方式增加病理学家评估子宫内膜增厚的频率。随着醋酸乌利司他剂量的增加,分泌分化以及亚核和核上空泡形成的频率有所下降[7]。
|
| 参考文献 |
[1]. Jadav SP, et al. Ulipristal acetate, a progesterone receptor modulator for emergency contraception. J Pharmacol Pharmacother. 2012 Apr;3(2):109-11.
[2]. Del Bello B, et al. Autophagy up-regulation by ulipristal acetate as a novel target mechanism in the treatment of uterine leiomyoma: an in vitro study. Fertil Steril. 2019 Dec;112(6):1150-1159. [3]. Hild SA, et al. CDB-2914: anti-progestational/anti-glucocorticoid profile and post-coital anti-fertility activity in rats and rabbits. Hum Reprod. 2000 Apr;15(4):822-9. [4]. Ciarmela P, et al. Ulipristal acetate modulates the expression and functions of activin a in leiomyoma cells. Reprod Sci. 2014 Sep;21(9):1120-5. [5]. Attardi BJ, et al. In vitro antiprogestational/antiglucocorticoid activity and progestin and glucocorticoid receptor binding of the putative metabolites and synthetic derivatives of CDB-2914, CDB-4124, and mifepristone. J Steroid Biochem Mol Biol. [6]. Pohl O, et al. Carcinogenicity and chronic rodent toxicity of the selective progesterone receptor modulator ulipristal acetate. Curr Drug Saf. 2013 Apr;8(2):77-97. [7]. Pohl O, et al. A 39-week oral toxicity study of ulipristal acetate in cynomolgus monkeys. Regul Toxicol Pharmacol. 2013 Jun;66(1):6-12 |
| 其他信息 |
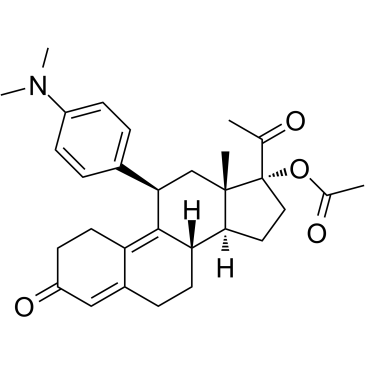
Ulipristal acetate is a 20-oxo steroid obtained by acetylation of the 17-hydroxy group of (11beta,17alpha)-17-acetyl-11-[4-(dimethylamino)phenyl]-3-oxoestra-4,9-dien-17-ol (ulipristal). A selective progesterone receptor modulator, which is employed as an emergency contraceptive. It has a role as a contraceptive drug, a progestin and a progesterone receptor modulator. It is a 3-oxo-Delta(4) steroid, a steroid ester, an acetate ester, a 20-oxo steroid and a tertiary amino compound. It is functionally related to an estradiol.
Ulipristal Acetate is an orally bioavailable, acetate salt of ulipristal, a selective progesterone receptor modulator with anti-progesterone activity. Ulipristal binds to the progesterone receptor (PR), thereby inhibiting PR-mediated gene expression, and interfering with progesterone activity in the reproductive system. As a result, this agent may suppress the growth of uterine leiomyomatosis. Furthermore, by inhibiting or delaying ovulation and effecting endometrial tissue, ulipristal can be used as an emergency contraception See also: Ulipristal (has active moiety). Drug Indication Emergency contraception within 120 hours (five days) of unprotected sexual intercourse or contraceptive failure. Ulipristal acetate is indicated for one treatment course of pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. Ulipristal acetate is indicated for intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age who are not eligible for surgery. Ulipristal acetate is indicated for pre-operative treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. Ulipristal acetate is indicated for intermittent treatment of moderate to severe symptoms of uterine fibroids in adult women of reproductive age. Contraception Leiomyoma of uterus |
| 分子式 |
C30H37NO4
|
|---|---|
| 分子量 |
475.6191
|
| 精确质量 |
475.272
|
| CAS号 |
126784-99-4
|
| 相关CAS号 |
Ulipristal;159811-51-5;Ulipristal acetate-d6;1621894-64-1
|
| PubChem CID |
130904
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
640.1±55.0 °C at 760 mmHg
|
| 熔点 |
183-185ºC
|
| 闪点 |
340.9±31.5 °C
|
| 蒸汽压 |
0.0±1.9 mmHg at 25°C
|
| 折射率 |
1.594
|
| LogP |
4.48
|
| tPSA |
63.68
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
35
|
| 分子复杂度/Complexity |
984
|
| 定义原子立体中心数目 |
5
|
| SMILES |
O(C(C([H])([H])[H])=O)[C@]1(C(C([H])([H])[H])=O)C([H])([H])C([H])([H])[C@@]2([H])[C@]3([H])C([H])([H])C([H])([H])C4=C([H])C(C([H])([H])C([H])([H])C4=C3[C@@]([H])(C3C([H])=C([H])C(=C([H])C=3[H])N(C([H])([H])[H])C([H])([H])[H])C([H])([H])[C@@]21C([H])([H])[H])=O
|
| InChi Key |
OOLLAFOLCSJHRE-ZHAKMVSLSA-N
|
| InChi Code |
InChI=1S/C30H37NO4/c1-18(32)30(35-19(2)33)15-14-27-25-12-8-21-16-23(34)11-13-24(21)28(25)26(17-29(27,30)3)20-6-9-22(10-7-20)31(4)5/h6-7,9-10,16,25-27H,8,11-15,17H2,1-5H3/t25-,26+,27-,29-,30-/m0/s1
|
| 化学名 |
[(8S,11R,13S,14S,17R)-17-acetyl-11-[4-(dimethylamino)phenyl]-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl] acetate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~33.33 mg/mL (~70.08 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.26 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.26 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1025 mL | 10.5126 mL | 21.0252 mL | |
| 5 mM | 0.4205 mL | 2.1025 mL | 4.2050 mL | |
| 10 mM | 0.2103 mL | 1.0513 mL | 2.1025 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Ulipristal Acetate for Use in Early Pregnancy Loss
CTID: NCT05216952
Phase: Phase 2 Status: Completed
Date: 2023-06-28