| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
JAK1 (IC50 = 43 nM); JAK2 (IC50 = 0.2 μM); JAK3 (IC50 = 2.3 μM); Tyk2 (IC50 = 4.7 μM)
|
|---|---|
| 体外研究 (In Vitro) |
在生化实验中,与参与红细胞生成的 JAK-2 相比,Upadacitinib 对 JAK-1 的选择性高出 74 倍,对 JAK-1 的选择性比参与免疫监视的 JAK-3 高 58 倍。 1]。由于 upadacitinib 对 JAK-1 的选择性优于 JAK-2 和 JAK-3,因此整个 RA 患者的获益-风险状况可能会得到改善 [2]。
Upadacitinib具有JAK1选择性,可抑制导致RA病理的细胞因子[3] 为了表征Upadacitini的酶活性,我们评估了利用重组人JAK激酶进行生化测定的效力和选择性。表1总结了数据。Upadacitinib显示出对JAK1(0.045μM)和JAK2(0.109μM)的活性 40 与JAK1相比,JAK3(2.1μM)的选择性是JAK3的100倍,TYK2(4.7μM)是JAK2的100倍。Upadacitinib还显示出对70+激酶的选择性,只有Rock1和Rock2的IC50值低于1μM(附加文件1:表S1)。为了进一步表征JAK1抑制upadactinib的机制,我们评估了不同浓度ATP下JAK1酶的活性。在所有测试浓度下,理论和实验IC50值的接近一致性证实了upadactinib是一种ATP竞争性抑制剂(数据未显示)。 Upadacitinib的JAK家族选择性在细胞检测中得到证实。由于JAK激酶合作性质的复杂性,我们采用了一组工程细胞系来了解upadactinib对每种激酶的细胞效力和选择性。如表1所示,upadactinib> 40 与JAK2(0.593μM)相比,JAK1(0.014μM)具有倍数选择性。Upadacitinib还显示出对JAK3(约130倍)和TYK2(约190倍)的选择性。还评估了upadactinib在生理相关细胞系统中的效力。与Ba/F3细胞数据一致,upadactinib能有效抑制JAK1依赖性细胞因子IL-6、OSM、IL-2和IFNγ,通过抑制STAT磷酸化来衡量。这项活动是~ 60 促红细胞生成素是一种完全依赖JAK2进行信号转导的细胞因子。接下来,我们测量了人全血中IL-6信号传导的抑制情况。upadactinib在CD3+T细胞群中的IC50值为0.207μM,在CD14+单核细胞群中为0.078μM。据报道,托法替尼对人全血中IL-6信号传导的IC50值对CD3+T细胞和单核细胞分别为0.367μM和0.406μM[3]。 |
| 体内研究 (In Vivo) |
在大鼠关节炎模型中,Upadacitinib(0.1-10 mg/kg;口服强饲;每天两次,持续 10 天)已证明有效[3]。ABT-494/Upadacitinib是大鼠AIA炎症和骨丢失的强效抑制剂,与托法替尼相比,在大鼠中以类似的有效剂量避免了相关的基本生理过程,如促红细胞生成素信号传导和外周NK细胞计数。在健康人类受试者中口服ABT-494 14天后,在与大鼠药效学特性一致的预测有效剂量下,ABT-494没有降低网织红细胞或NK细胞计数。
结论ABT-494是一种Jak1选择性抑制剂,在大鼠关节炎模型中显示出疗效。初步证据表明,ABT-494的药效学特性与在啮齿动物模型和健康人体受试者中观察到的一致。总的来说,这些令人鼓舞的观察结果支持在II期随机安慰剂对照试验中在RA患者中进一步测试ABT-494,并表明它可能比现有药物更有可能满足患者的需求。[2] Upadacitinib抑制大鼠佐剂性关节炎的疾病病理[3] 为了了解对炎症和关节炎表型的影响,我们在佐剂诱导的关节炎模型中测试了Upadacitinib,这是一种已建立的RA临床前模型。在第7天出现疾病的最初症状时口服乌帕西替尼,导致爪肿胀的剂量和暴露依赖性减少(图2a)。在疾病诱导后第18天,收获爪子,通过μCT测量骨破坏情况。AIA的正常过程会导致骨体积的显著减少,并且随着upadactinib的给药,骨体积会呈剂量依赖性减少(图2b)。与骨表面受到保护的10mg/kg upadacitnib治疗动物(图2d)相比,经载体处理的动物(图2c)出现了明显的凹陷和骨丢失,显示了破坏的例子。本研究还评估了组织学终点。Upadacitinib给药改善了3和10mg/kg剂量组的滑膜肥大、炎症、软骨损伤和骨侵蚀(数据未显示)。在大鼠胶原诱导性关节炎(CIA)模型中也观察到了类似的结果,这是RA的第二个临床前模型(数据未显示)。托法替尼也在AIA模型中进行了测试,并证明了剂量反应性疗效,尽管与upadactinib相比,暴露反应曲线右移(图2a)。有效浓度定义为达到60%抑制爪肿胀所需的AUC0-12药物浓度(AUC60)。使用AUC60作为参考点的理由是基于与10 mg BID临床剂量的托法替尼相关的AUC暴露量。这为进一步分析建立了一个翻译参考点。计算出乌帕替尼的总有效药物暴露量为83 ng*hr./ml,而托法替尼的暴露量为1205 ng*hr./ml。根据JAK1细胞效力与托法替尼的差异,预计乌帕替尼的体内效力会增加。 与疗效相比,Upadacitinib可避免网织红细胞部署和NK细胞计数减少[3] 我们应用了一种先前描述的方法的变体,以确定Upadacitini和托法替尼对JAK2依赖性Epo受体功能抑制的相对影响。连续两天用PBS或1000IU的Epo静脉注射幼年大鼠,并在第4天测量循环网织红细胞。始终给药upadacinib或tofactinib,并通过流式细胞术定量网织红细胞。我们还试图确定upadactinib和tofacitinib对循环NK细胞计数形式的常见γ链信号传导(JAK1/JAK3)的影响,因为这些细胞依赖IL-15生存。新生大鼠服用乌帕替尼或托法替尼14天,通过流式细胞术定量循环CD3-/CD16+/CD56+NK细胞。将网织红细胞部署、循环NK细胞计数和AIA疗效实验的结果一起绘制,以显示与暴露相关的相对影响(图3)。托法替尼以暴露依赖的方式(AUC60为1230 ng*hr./ml)降低循环NK细胞数量,类似于观察到的抑制爪子肿胀的暴露范围(AUC60 = 1205 ng*hr./ml)。托法替尼以暴露依赖的方式减少网织红细胞部署,达到最大抑制> 40% 在测试的最高浓度下(图3a)。Upadacitinib与AUC60以暴露依赖的方式降低循环NK细胞数量 = 480 ng*hr.ml~ 5 比抑制爪子肿胀所需的药物浓度(AUC60)低一倍 = 83 ng*hr./ml)。网织红细胞的部署也呈剂量反应性减少,并达到最大抑制作用~ 40% (图3b)。在与10 mg BID的托法替尼相关的临床AUC暴露量下,爪子肿胀的减少~ 60% 并且与NK细胞耗竭有明显的重叠(图3a)。使用6 mg BID和12 mg BID临床剂量的乌帕西替尼,爪肿胀的减少> 90%,与NK细胞耗竭明显分离(图3b)。 在AIA模型中,网织红细胞(图4a)和NK细胞(图4b)数据与爪肿胀抑制率进行了重新绘制,以直接比较托法替尼和Upadacitinib的抑制作用与疾病疗效的关系。在较低的有效范围内,托法替尼和乌帕替尼对网织红细胞的相对影响相似,但在较高的有效范围(>60%的爪肿胀)内,差异效应变得更加明显(图4a)。同样,循环NK细胞计数也有明显的差异效应。在AUC60时,托法替尼治疗后循环NK细胞减少了70%,而乌帕替尼治疗导致减少了25%(图4b)。 Upadacitinib在健康志愿者中,相对于IL-6信号传导,保留了常见的γ链信号传导[3] 为了确认Upadacitinib在临床环境中的临床前选择性,在服用1、3、12、24、36或48 mg Upadacitinib或5 mg tofacitinib的健康志愿者的全血中进行了离体细胞因子刺激试验。给药后1小时,抽血并用IL-6或IL-7刺激,以评估upadactinib对这些信号通路的影响。通过流式细胞术评估下游STAT磷酸化(STAT3和STAT5)的抑制作用(图5)。JAK1介导的IL-6诱导的pSTAT3被抑制~ 50% 在3mg剂量的upadactinib下,相当于5mg托法替尼的抑制水平。在36 mg达到最大抑制之前,增加剂量的乌帕西替尼显示了pSTAT3抑制的伴随增加。为了评估体内JAK1/3的效力,使用IL-7驱动的pSTAT5评估了抗常见γ链信号传导的活性。在这种情况下,需要12mg的乌帕替尼才能将pSTAT5抑制到与5mg的托法替尼相同的程度(约70%)。 |
| 酶活实验 |
酶效力和选择性测定[3]
JAK1(aa 845-1142)和JAK3(aa 811-1103)的活性重组人催化结构域在内部制备,并在SF9细胞中表达为谷胱甘肽s转移酶(GST)融合物,并通过谷胱甘肽亲和层析纯化。活性人TYK2(aa880-1185)在室内纯化,含有N端组氨酸标签和C端FLAG标签。通过固定化金属离子亲和层析进行纯化。JAK2的重组激酶结构域购自xxx。使用肽生物素-TYR2(生物素-(Ahx)-AEEYFFLFA酰胺)和生物素-TYR1。在抑制剂和2μM肽的存在下,在100μM ATP下进行反应。对于竞争试验,在不同量的ATP(0.01-1mM)等于或大于激酶的ATP-Km的情况下,测定Upadacitini的JAK1 IC50。使用Cheng-Prusoff方程评估ATP竞争力。ATP竞争性抑制剂在不同ATP浓度下的IC50变化与Cheng-Prusoff方程得出的理论值一致。 |
| 细胞实验 |
Ba/F3细胞效力和选择性测定[3]
TEL-JAK2、TEL-JAK3、TEL-TYK2和BCR-JAK1-Ba/F3工程细胞系购自Advanced Cellular Dynamics。细胞在添加了10%胎牛血清、1×青霉素-链霉素-谷氨酰胺和0.5μg/ml嘌呤霉素的RPMI 1640培养基中生长。 为了测量信号转导子和转录激活子5(pSTAT5)的磷酸化,将细胞洗涤并重新悬浮在密度为2 X 107个细胞/mL的Hank's平衡盐溶液中。将5微升细胞悬浮液加入到含有5μL化合物的384孔、低体积、白壁聚苯乙烯板中(在11点[1:3]滴定系列中)。在进行pSTAT5检测之前,细胞在37°C下与化合物(最终DMSO浓度0.5%)一起孵育30分钟。按照标准制造商的方案,使用SureFire pSTAT5检测试剂盒测量pSTAT5,但在EnVision上检测之前,在添加供体珠后进行过夜温育除外。 细胞因子效力测定[3] 在人红白血病TF-1细胞系中评估了IL-6和肿瘤抑素M(OSM)诱导的STAT3磷酸化。在人UT-7细胞系中评估了促红细胞生成素诱导的STAT5磷酸化。在活化的人T细胞中评估IL-2和IL-15诱导的STAT5磷酸化。根据标准制造商的方案,使用SureFire pSTAT5或pSTAT3检测试剂盒完成磷酸化STAT的检测,但在EnVision上检测之前,在添加供体珠后进行过夜孵育除外。通过流式细胞术评估人PBMC中CD14+单核细胞群中IFNγ诱导的STAT1磷酸化。使用CD14 BV421和STAT1-PE(pY705)。通过流式细胞术评估成人上皮角质形成细胞中IL-4和IL-13诱导的STAT6磷酸化和IL-31诱导的STAT3磷酸化。使用STAT6-PE(pY641)和STAT3-PE(Y705)。 |
| 动物实验 |
Animal/Disease Models: Female Lewis rat (rat adjuvant-induced arthritis model) [3]
Doses: 0.1, 0.3, 1, 3, 10 mg/kg Route of Administration: po (oral gavage); twice a day for 10 days Experimental Results: Inhibition of disease pathology in adjuvant-induced arthritis in rats. The efficacy and selectivity of Upadacitinib/ABT-494 were tested in a battery of relevant cellular and in vivo pharmacology assays including bone marrow colony formation, adjuvant induced arthritis (AIA), erythropoietin induced reticulocyte deployment and NK/NKT cell suppression. The potency of ABT-494 in a variety of complementary pharmacodynamic assays was also assessed at multiple dosages in healthy human subjects administered orally for 14 days. [2] Rat adjuvant-induced arthritis (AIA) model [3] Arthritis was induced in female Lewis rats (weight, 125 – 150 g) by a single intradermal injection of 0.1 mL of microbacterium tuberculosis emulsion into the right hind footpad (Day 0). Rats were dosed as indicated orally by gavage twice a day (BID) for 10 days (Day7 – Day17) post immunization with either vehicle or study drug. To evaluate the severity of arthritis, paw swelling was evaluated with a water displacement plethysmograph every other day up to Day 17. On Day 17, all rats were exsanguinated by cardiac puncture under isolfuorane anesthesia. Left rear paws were scanned using a μCT. Bone volume and density were determined in a 360 μm vertical section encompassing the tarsal section of the paw. Reticulocyte deployment assays Naïve male Lewis rats were injected intravenously with either PBS or 1000 IU of epoetin α for two consecutive days. Reticulocytes were measured on day 4 by flow cytometry using thiozole orange as a dye as previously described [13]. Dose responses of either Upadacitinib or tofacitinib were dosed 30 min prior to the first Epo injection and then once every 12 h subsequently for 3 days. NK cell analysis [3] Sprague Dawley rats were dosed orally with either Upadacitinib or tofacitinib at doses indicated for 14 days. Blood was collected and stained using BD MultiTest IMK kit per manufacturer’s instructions. NK cell numbers were determined by using FlowJo analysis software and by examining the CD3−/CD16+/CD56+ population. The number of cells/μL was calculated by using the following equation: (# events in cell population/# of events in absolute bead count region) × (# beads per test/test volume), with the value beads per test indicated on the BD Trucount tube label. Pharmacokinetic/pharmacodynamics modeling [3] A direct maximum enhancement model was the most predictive for defining the efficacious concentration range and human efficacious dose. Efficacious area under the concentration-versus-time curve (AUC) was based on paw swelling on the last day of the study plotted against the cumulative plasma concentration of Upadacitinib or tofacitinib over 12 h (AUC0–12). Clinical ex vivo stimulation assays [3] For each subject, blood was collected by venipuncture into 2 mL sodium heparin tubes at 0, 1, 6, and 12 h post Upadacitinib or tofacitinib dose. Recombinant human IL-6 (400 ng/ml), or IL-7 (400 ng/ml), was added to blood and incubated for 10 min at 37°C. Surface antibodies were added (CD14-APC, CD3-fluorescein isothiocyanate [FITC]) and incubated on ice for an additional 20 min. Samples were lysed and incubated for 10 min at 37°C. Samples were washed and stored at − 70°C. For intracellular staining, samples were thawed, washed, and resuspended in BD Perm buffer III on ice for 30 min. Samples were washed and stained with pSTAT5-PE or pSTAT3-PE for 60 min at room temperature and then analyzed immediately on a FACSCalibur. Geometric means were determined using FlowJo analysis software. Percent inhibition of relevant STAT phosphorylation was calculated as follows: (1-(Induction of pSTAT at 1 h – baseline pSTAT at 0 h) / (Induction of pSTAT at 0 h – baseline pSTAT at 0 h)*100. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Upadacitinib displays a dose-proportional pharmacokinetic profile over the therapeutic dose range. Following oral administration, the median time to reach Cmax (Tmax) ranges from 2 to 4 hours. The steady-state plasma concentrations of upadacitinib are reached within 4 days following multiple once-daily administrations, with minimal accumulation. Food intake has no clinically relevant effect on the AUC, Cmax, and Cmin of upadacitinib from the extended-release formulation. Following administration of a single radio-labelled dose from the immediate-release formulation, approximately 53% of the total dose was excreted in the feces where 38% of the excreted dose was an unchanged parent drug. About 43% of the total dose was excreted in the urine, where 24% of that dose was in the unchanged parent drug form. Approximately 34% of the total dose of upadacitinib dose was excreted as metabolites. The volume of distribution of upadacitinib in a patient with rheumatoid arthritis and a body weight of 74 kg is estimated to be 224 L following oral administration of an extended-release formula. In a pharmacokinetic study consisting of healthy volunteers receiving the extended-release formulation, the steady-state volume of distribution was 294 L. Upadacitinib partitions similarly between plasma and blood cellular components with a blood to plasma ratio of 1.0. The apparent oral clearance of upadacitinib in healthy volunteers receiving the extended-release formulation was 53.7 L/h. Metabolism / Metabolites Upadacitinib predominantly undergoes CYP3A4-mediated metabolism; however, upadacitinib is a nonsensitive substrate of CYP3A4. It is also metabolized by CYP2D6 to a lesser extent. In a human radio-labelled study, about 79% of the total plasma radioactivity accounted for the parent drug, and about 13% of the total plasma radioactivity accounted for the main metabolite produced from mono-oxidation, followed by glucuronidation. There are no known active metabolites of upadacitinib. Biological Half-Life The mean terminal elimination half-life of upadacitinib ranged from 8 to 14 hours following administration of the extended-release formulation. In clinical trials, approximately 90% of upadacitinib in the systemic circulation was eliminated within 24 hours of dosing. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In the prelicensure clinical trials of upadacitinib in patients with rheumatoid arthritis, liver test abnormalities were frequent although usually mild. Some degree of ALT elevation arose in up to 11% of upadacitinib treated patients compared to 7% treated with placebo, but were above 3 times the upper limit of normal (ULN) in 2% or less. Furthermore, similar rates of ALT elevations arose in patients treated with methotrexate or biologic DMARDs. In these trials that enrolled over 3000 patients, there were no reports of clinically apparent liver injury, serious cases of liver injury or liver-related deaths. Similarly, other JAK inhibitors such as tofacitinib and baricitinib have been associated with frequent minor serum aminotransferase elevations during treatment, but episodes of clinically apparent liver injury have not been reported. Thus, this class of agents is suspected but not proven capable of causing liver injury. In addition, long term treatment with upadacitinib and other Janus kinase inhibitors has been linked to rare instances of reactivation of hepatitis B that can be severe and has been linked to fatal outcomes. Reactivation can become clinically apparent after the JAK inhibitor is discontinued, when immune restoration results in an immunologic response to the heightened viral replication. Likelihood score: D (possible, rare cause of clinically apparent liver injury including reactivation of hepatitis B in susceptible patients). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of upadacitinib during breastfeeding. Most sources recommend that mothers not breastfeed while taking upadacitinib. An alternate drug is preferred, especially while nursing a newborn or preterm infant. The manufacturer recommends that breastfeeding be withheld for 6 days after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Upadacitinib is 52% bound to human plasma proteins. Toxicity Summary Upadacitinib was found to be teratogenic in animal studies, although no human studies during pregnancy have been reported. Use during pregnancy is not recommended. Contraception is advised during treatment and for 4 weeks after completing treatment with upadacitinib. Hepatotoxicity The pattern of liver injury associated with upadacitinib indicates a potential for low-level, direct hepatotoxicity. During the initial weeks of treatment, ALT levels may slightly increase, typically returning to baseline upon discontinuation of the drug. Serum aminotransferase elevations above 5 times the upper limit of normal should prompt consideration of dose reduction or temporary cessation of upadacitinib treatment. Managing potential hepatotoxicity carefully is essential for minimizing further liver injury. Close monitoring of liver function tests is crucial during this period to ensure timely intervention and appropriate management. There is no information regarding overdose of upadacitinib in the FDA-approved product labeling. Adverse Effects Upadacitinib has various adverse effects, which are listed below. * Upper respiratory tract infections (URTI) (14%) * Nausea (4%) * Elevated liver enzymes (2%) * Fever (1%) * Cough (2%) Upper respiratory tract infections (URTI) encompass: * Acute sinusitis * Laryngitis * Nasopharyngitis * Oropharyngeal pain * Pharyngitis * Pharyngotonsillitis * Rhinitis * Sinusitis * Tonsillitis * Viral upper respiratory tract infection These adverse effects were observed during placebo-controlled studies where subjects were administered 15 mg of oral upadacitinib. More severe adverse effects, such as herpes zoster virus (HZV) and serious infections, were seen in subjects administered 30 mg in a double-blind, randomized, controlled phase 3 clinical trial (Moraxella infection. Drug-Drug Interactions: Upadacitinib is metabolized in the liver by the cytochrome P450 (CYP) system, primarily through the CYP3A4 enzyme, and is eliminated in the feces and urine as metabolites, with a drug half-life of 8 to 14 hours. The concomitant use of CYP3A4 inhibitors and CYP3A4 inducers should be approached with caution and is generally not recommended, as it may alter the drug's pharmacokinetics, potentially increasing or decreasing drug plasma concentrations. Clinical studies have also reported malignancy, thrombosis, and gastrointestinal (GI) perforations with concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs). * Upadacitinib exposure may increase when used concurrently with strong CYP3A4 inhibitors such as grapefruit, ketoconazole, and clarithromycin, potentially exacerbating the risk of adverse reactions. Patients should avoid consuming grapefruit or grapefruit-containing products during upadacitinib treatment. * For patients with atopic dermatitis or Crohn disease who are prescribed strong CYP3A4 inhibitors, the induction dosage of upadacitinib should be reduced to 30 mg once daily while maintaining the maintenance dosage at 15 mg once daily. Conversely, strong CYP3A4 inducers such as rifampin may decrease upadacitinib exposure, potentially diminishing its therapeutic effects. Therefore, co-administration of upadacitinib with strong CYP3A4 inducers is not advised. |
| 参考文献 |
|
| 其他信息 |
Pharmacodynamics
Upadacitinib is a DMARD that works by inhibiting the Janus Kinases (JAKs), which are essential downstream cell signalling mediators of pro-inflammatory cytokines. It is believed that these pro-inflammatory cytokines play a role in many autoimmune inflammatory conditions, such as rheumatoid arthritis. In clinical trials, upadacitinib decreased the activity of pro-inflammatory interleukins, transiently increased the levels of lymphocytes, and insignificantly decreased the levels of immunoglobulins from the baseline. Upadacitinib is an oral Janus kinase (JAK)1-selective inhibitor and a disease-modifying antirheumatic drug (DMARD) used in the treatment of rheumatoid arthritis to slow down disease progression. Rheumatoid arthritis is a chronic autoimmune inflammatory disease affecting the peripheral joints. It is characterized by synovial inflammation and hyperplasia, autoantibody production, cartilage damage and bone destruction, leading to co-morbidities. Despite a variety of therapeutic agents available for treatment, up to 40% of the patients do not respond to current therapies, including biological therapies. The etiology of the disease is mostly unknown; however, the role of JAK as a driver of immune-mediated conditions was discovered, leading to the use of JAK as therapeutic targets for rheumatoid arthritis. To reduce dose-related toxicity (as seen with some pan-JAK inhibitors) without significantly affecting efficacy, more selective JAK1 inhibitors, upadacitinib and [filgotinib], were developed. The FDA approved upadacitinib in August 2019 and it is used for the treatment of active rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, ulcerative colitis, and ankylosing spondylitis. In December 2019, it was additionally approved by the European Commission and Health Canada. Upadacitinib is marketed under the brand name RINVOQ for oral administration. Upadacitinib is a Janus Kinase Inhibitor. The mechanism of action of upadacitinib is as a Janus Kinase Inhibitor. Upadacitinib is an oral selective inhibitor of Janus associated kinase 1 (JAK-1) that is used in the therapy of moderate-to-severe rheumatoid arthritis. Upadacitinib has been associated with a low rate of serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent acute liver injury although it may pose a risk for reactivation of hepatitis B in susceptible patients. Upadacitinib is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2019 and is indicated for rheumatoid arthritis and has 12 investigational indications. This drug has a black box warning from the FDA. Drug Indication Upadacitinib is indicated for the treatment of moderately to severely active **rheumatoid arthritis** or active **psoriatic arthritis** in adult patients who have had an inadequate response or intolerance to one or more disease-modifying anti-rheumatic drugs (DMARDs), such as TNF blockers. In Europe, upadacitinib may be used as monotherapy or in combination with [methotrexate] for rheumatoid or psoriatic arthritis. Upadacitinib is indicated for use in patients 12 years of age and older with refractory, moderate-to-severe **atopic dermatitis** whose disease is inadequately controlled with other systemic therapies or when other therapies are inadvisable. Upadacitinib is indicated for the treatment of active **ankylosing spondylitis** or radiographic axial spondyloarthritis in adult patients who have an inadequate response to conventional therapy. It is also indicated to treat non-radiographic axial spondyloarthritis with objective signs of inflammation in adults who have had an inadequate response or intolerance to TNF blocker therapy. Upadacitinib is also indicated to treat moderately to severely active **ulcerative colitis** in adults who have had an inadequate response or intolerance to either conventional therapy or a biologic agent, such as to one or more TNF blockers. Upadacitinib is indicated to treat moderately to severely active Crohn’s disease in adults who have had an inadequate response or intolerance to one or more TNF blockers. Combining upadacitinib with other JAK inhibitors, biologic DMARDs, or other potent immunosuppressive agents is not recommended. Rheumatoid arthritis RINVOQ is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs (DMARDs). RINVOQ may be used as monotherapy or in combination with methotrexate. Psoriatic arthritis RINVOQ is indicated for the treatment of active psoriatic arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more DMARDs. RINVOQ may be used as monotherapy or in combination with methotrexate. Axial spondyloarthritis Non-radiographic axial spondyloarthritis (nr-axSpA)RINVOQ is indicated for the treatment of active non-radiographic axial spondyloarthritis in adult patients with objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI), who have responded inadequately to nonsteroidal anti-inflammatory drugs (NSAIDs). Ankylosing spondylitis (AS, radiographic axial spondyloarthritis )RINVOQ is indicated for the treatment of active ankylosing spondylitis in adult patients who have responded inadequately to conventional therapy. Atopic dermatitisRINVOQ is indicated for the treatment of moderate to severe atopic dermatitis in adults and adolescents 12 years and older who are candidates for systemic therapy. Ulcerative colitisRINVOQ is indicated for the treatment of adult patients with moderately to severely active ulcerative colitis who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent. Â Crohn's diseaseRINVOQ is indicated for the treatment of adult patients with moderately to severely active Crohn's disease who have had an inadequate response, lost response or were intolerant to either conventional therapy or a biologic agent. Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by synovial inflammation and joint destruction. Considerable advance in the treatment of RA has been made following the advent of biological disease-modifying anti-rheumatic drugs (DMARDs). However, these biologics require intravenous or subcutaneous injection and some patients fail to respond to biological DMARDs or lose their primary response. Various cytokines and cell surface molecules bind to receptors on the cell surface, resulting in the activation of various cell signaling pathways, including phosphorylation of kinase proteins. Among these kinases, the non-receptor tyrosine kinase family Janus kinase (JAK) plays a pivotal role in the pathological processes of RA. Several JAK inhibitors have been developed as new therapies for patients with RA. These are oral synthetic DMARDs that inhibit JAK1, 2, and 3. One JAK inhibitor, tofacitinib, has already been approved in many countries. Results of phase III clinical trials using a JAK1/2 inhibitor, baricitinib, have shown feasible efficacy and tolerable safety. Both drugs are effective in patients who showed inadequate response to biological DMARDs as well as synthetic DMARDs. In addition, clinical phase III trials using filgotinib and Upadacitinib/ABT-494, specific JAK1 inhibitors, are currently underway. JAK inhibitors are novel therapies for RA, but further studies are needed to determine their risk-benefit ratio and selection of the most appropriate patients for such therapy. [1] Anti-cytokine therapies have become the mainstay of treatment for rheumatoid arthritis (RA) disease symptoms and can arrest disease progression. Despite numerous treatment options there are still many RA patients who fail to experience substantial decreases in disease activity. Recently, Jak kinase blockade was shown clinically to be effective in managing disease and in some cases achieving remission. However, these first generation Jak inhibitors have failed to meet expectations due to dose-limiting tolerability and safety issues. ABT-494 is a second generation Jak kinase inhibitor with high selectivity for Jak1 thereby minimizing the potential for side effects related to Jak2 and Jak3 inhibition. Here we describe preclinical and early clinical data that suggest ABT-494 has potential to address some of the current unmet medical needs of RA patients.[2] Background: Anti-cytokine therapies such as adalimumab, tocilizumab, and the small molecule JAK inhibitor tofacitinib have proven that cytokines and their subsequent downstream signaling processes are important in the pathogenesis of rheumatoid arthritis. Tofacitinib, a pan-JAK inhibitor, is the first approved JAK inhibitor for the treatment of RA and has been shown to be effective in managing disease. However, in phase 2 dose-ranging studies tofacitinib was associated with dose-limiting tolerability and safety issues such as anemia. Upadacitinib (ABT-494) is a selective JAK1 inhibitor that was engineered to address the hypothesis that greater JAK1 selectivity over other JAK family members will translate into a more favorable benefit:risk profile. Upadacitinib selectively targets JAK1 dependent disease drivers such as IL-6 and IFNγ, while reducing effects on reticulocytes and natural killer (NK) cells, which potentially contributed to the tolerability issues of tofacitinib. Methods: Structure-based hypotheses were used to design the JAK1 selective inhibitor Upadacitinib. JAK family selectivity was defined with in vitro assays including biochemical assessments, engineered cell lines, and cytokine stimulation. In vivo selectivity was defined by the efficacy of upadacitinib and tofacitinib in a rat adjuvant induced arthritis model, activity on reticulocyte deployment, and effect on circulating NK cells. The translation of the preclinical JAK1 selectivity was assessed in healthy volunteers using ex vivo stimulation with JAK-dependent cytokines. Results: Here, we show the structural basis for the JAK1 selectivity of Upadacitinib, along with the in vitro JAK family selectivity profile and subsequent in vivo physiological consequences. Upadacitinib is ~ 60 fold selective for JAK1 over JAK2, and > 100 fold selective over JAK3 in cellular assays. While both upadacitinib and tofacitinib demonstrated efficacy in a rat model of arthritis, the increased selectivity of upadacitinib for JAK1 resulted in a reduced effect on reticulocyte deployment and NK cell depletion relative to efficacy. Ex vivo pharmacodynamic data obtained from Phase I healthy volunteers confirmed the JAK1 selectivity of upadactinib in a clinical setting. Conclusions: The data presented here highlight the JAK1 selectivity of Upadacitinib and supports its use as an effective therapy for the treatment of RA with the potential for an improved benefit:risk profile. |
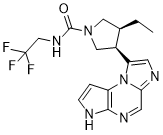
| 分子式 |
C17H19F3N6O
|
|---|---|
| 分子量 |
380.367573022842
|
| 精确质量 |
380.157
|
| 元素分析 |
C, 53.68; H, 5.04; F, 14.98; N, 22.09; O, 4.21
|
| CAS号 |
1310726-60-3
|
| 相关CAS号 |
Upadacitinib-15N,d2;Upadacitinib tartrate tetrahydrate;1607431-21-9
|
| PubChem CID |
58557659
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 熔点 |
16-19
|
| 折射率 |
1.678
|
| LogP |
3.06
|
| tPSA |
78.3
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
561
|
| 定义原子立体中心数目 |
2
|
| SMILES |
CC[C@@H]1CN(C[C@@H]1C2=CN=C3N2C4=C(NC=C4)N=C3)C(=O)NCC(F)(F)F
|
| InChi Key |
WYQFJHHDOKWSHR-MNOVXSKESA-N
|
| InChi Code |
InChI=1S/C17H19F3N6O/c1-2-10-7-25(16(27)24-9-17(18,19)20)8-11(10)13-5-22-14-6-23-15-12(26(13)14)3-4-21-15/h3-6,10-11,21H,2,7-9H2,1H3,(H,24,27)/t10-,11+/m1/s1
|
| 化学名 |
(3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide
|
| 别名 |
ABT-494; Upadacitinib; ABT-494; Rinvoq; Upadacitinib anhydrous; UNII-4RA0KN46E0; 4RA0KN46E0; ABT 494; ABBV-599 COMPONENT UPADACITINIB; ABT494
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~262.90 mM)
H2O : < 0.1 mg/mL |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.75 mg/mL (7.23 mM) (饱和度未知) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.75 mg/mL (7.23 mM) (饱和度未知) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 1.67 mg/mL (4.39 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: ≥ 1.67 mg/mL (4.39 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100μL 16.7mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: ≥ 1.67 mg/mL (4.39 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 16.7 mg/mL 澄清 DMSO 储备液加入900 μL 玉米油中,混合均匀。 配方 6 中的溶解度: (饱和度未知) in (这些助溶剂从左到右依次添加,逐一添加), *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.6290 mL | 13.1451 mL | 26.2902 mL | |
| 5 mM | 0.5258 mL | 2.6290 mL | 5.2580 mL | |
| 10 mM | 0.2629 mL | 1.3145 mL | 2.6290 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Assess Change in Disease Activity and Adverse Events of Oral Upadacitinib in Adult and Adolescent Participants With Moderate to Severe Hidradenitis Suppurativa Who Have Failed Anti-TNF Therapy
CTID: NCT05889182
Phase: Phase 3 Status: Recruiting
Date: 2024-11-25