| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| 25g |
|
||
| 50g |
|
||
| Other Sizes |
|

| 靶点 |
HDAC1 ( IC50 = 400 μM ); HDAC1 ( IC50 = 0.5-2 mM ); HDAC2; Autophagy; Mitophagy
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:丙戊酸通过一种独特的途径发挥作用,该途径涉及直接抑制组蛋白脱乙酰酶(HDAC1 的 IC(50) = 0.4 mM)。丙戊酸模仿组蛋白脱乙酰酶抑制剂曲古抑菌素 A,导致培养细胞中组蛋白过度乙酰化。丙戊酸与曲古抑菌素 A 一样,也可激活多种外源和内源启动子的转录。丙戊酸和曲古抑菌素 A 在脊椎动物胚胎中具有非常相似的致畸作用,而丙戊酸的非致畸类似物不会抑制组蛋白脱乙酰酶,也不会激活转录。丙戊酸诱导啮齿动物肝脏中过氧化物酶体的增殖。在表达与糖皮质激素受体 (GR) 的 DNA 结合结构域融合的 PPARδ 配体结合结构域的细胞系中,浓度为 1 mM 的丙戊酸可通过 N-CoR、TR 或 PPARδ 的 Gal4 融合来缓解这种抑制与 GR 控制的报告基因一起。丙戊酸诱导高乙酰化组蛋白的积累并抑制 HDAC 活性。丙戊酸诱导特定类型的分化,其特征是增殖减少、形态改变、标记基因表达,特别是 AP-2 转录因子的积累,作为 F9 畸胎癌细胞中神经元或神经嵴细胞样分化的潜在标记。 F9 和 P19 畸胎癌细胞中[3H]胸苷掺入减少表明丙戊酸会损害细胞增殖或存活。激酶测定:分别使用 caspase-3、-8 和 -9 比色测定试剂盒评估 caspase-3、-8 和 -9 的活性。简而言之,将 60 mm 培养皿中的 1×106 个细胞与 10 mM 丙戊酸一起孵育 24 小时。然后将细胞在 PBS 中洗涤并悬浮在试剂盒随附的 5 倍体积的裂解缓冲液中。使用布拉德福德法测定蛋白质浓度。含有 50 μg 总蛋白的上清液用于测定 caspase-3、-8 和 -9 活性。将上清液添加到以 DEVD-pNA、IETD-pNA 或 LEHD-pNA 作为 caspase-3、-8 和 -9 底物的 96 孔微量滴定板的每个孔中,并将板在 37°C 下孵育 1 小时。使用酶标仪在 405 nm 处测量每个孔的光密度。 caspase-3、-8 和 -9 的活性以任意吸光度单位表示。细胞测定:简而言之,将 5×105 个细胞接种到 96 孔微量滴定板中进行 MTT 测定。在暴露于指定剂量的丙戊酸指定时间后,将 MTT 溶液 [20 mL: 2 mg/mL,溶于磷酸盐缓冲盐水 (PBS)] 添加到 96 孔板的每个孔中。将板另外在 37°C 下孵育 3 小时。通过移液从板中抽出培养基,并向每孔中添加 200 mL DMSO 以溶解甲臜晶体。使用酶标仪在 570 nm 处测量光密度。
|
||
| 体内研究 (In Vivo) |
Valproic Acid (500 mg/kg, ip) 抑制移植 Kasumi-1 细胞的小鼠的肿瘤生长和血管生成。实验结束时丙戊酸组的IR率为57.25%。丙戊酸(350 mg/kg,腹腔注射)比对照动物表现出更多的社会调查和打斗游戏
|
||
| 酶活实验 |
caspase-3、-8 和 -9 的比色测定试剂盒分别用于测量这些酶的活性。简而言之,10 mM 丙戊酸与 1×106 个细胞在 60 mm 培养皿中孵育 24 小时。 PBS 洗涤后,将细胞悬浮在五体积的试剂盒裂解缓冲液中。布拉德福德法用于测定蛋白质浓度。在含有 50 μg 总蛋白的上清液中测量 caspase-3、-8 和 -9 的活性。在含有 caspase-3、-8 或 -9 底物(DEVD-pNA、IETD-pNA 或 LEHD-pNA)的 96 孔微量滴定板中,将上清液添加到每个孔中。然后将板在 37°C 下孵育一小时。使用酶标仪,在 405 nm 处测定每个孔的光密度。任意吸光度单位用于表示 caspase-3、-8 和 -9 的活性。
|
||
| 细胞实验 |
对于 MTT 测定,将 5×105 细胞接种到 96 孔微量滴定板中。 96 孔板的每个孔中装有 20 mL 的 MTT 溶液(2 mg/mL 磷酸盐缓冲盐水;PBS),该溶液已暴露于规定剂量的丙戊酸指定的时间。此外,将板在 37°C 下孵育三个小时。为了溶解甲臜晶体,将培养基从板中移出后,向每个孔中添加 200 mL DMSO。通过使用酶标仪,在 570 nm 处测量光密度。
|
||
| 动物实验 |
|
||
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Prospective studies suggest that 5% to 10% of persons develop ALT elevations during long term valproate therapy, but these abnormalities are usually asymptomatic and can resolve even with continuation of drug. Unlike phenytoin and carbamazepine, valproate does not induce elevations in serum GGT levels. More importantly and not uncommonly, valproate can cause several forms of clinically apparent hepatotoxicity. Indeed, more than 100 fatal cases of acute or chronic liver injury due to valproate have been reported in the literature. Three clinically distinguishable forms of hepatotoxicity (besides simple aminotransferase elevations) can occur with valproate. The first syndrome is hyperammonemia with minimal or no evidence of hepatic injury. This syndrome typically presents with progressive and episodic confusion followed by obtundation and coma. The time to onset is often within a few weeks of starting valproate or increasing the dose, but it can present months or even years after starting the medication (Case 1). The diagnosis is made by the finding of elevations in serum ammonia with normal (or near normal) serum aminotransferase and bilirubin levels. Valproate levels are usually normal or minimally high. The syndrome resolves within a few days of stopping valproate, but may reverse more rapidly with carnitine supplementation or renal hemodialysis. The second form of injury from valproate is an acute hepatocellular injury with jaundice, typically accompanied by hepatocellular or mixed pattern of enzyme elevations (Case 2). This acute liver injury pattern usually has its onset within 1 to 6 months of starting valproate. The pattern of serum enzyme elevations can be hepatocellular or mixed; sometimes the serum aminotransferase levels are not markedly elevated, despite the severity of injury. Immunoallergic features (fever, rash, eosinophilia) are usually absent, but rare cases with prominent features of hypersensitivity have been reported (Case 3). Multiple instances of fatal acute hepatic failure due to valproate have been published and valproate is regularly listed as a cause of drug induced acute liver failure. Liver histology is distinctive and reveals a microvesicular steatosis with central lobular necrosis, mild to moderate inflammation and cholestasis. In cases with a prolonged course, fibrosis, bile duct proliferation and regenerative nodules may be present. Prospective studies using historical controls suggest that carnitine (particularly intravenously) may be beneficial if given soon after presentation. The third form of hepatic injury due to valproate is a Reye-like syndrome described in children on valproate who develop fever and lethargy (suggestive of a viral infection) followed by confusion, stupor and coma, with raised ammonia levels and marked ALT elevations but normal or minimally elevated bilirubin levels. Metabolic acidosis is also common and the syndrome can be rapidly fatal. Valproate may simply be an aspirin-like agent capable of triggering Reye syndrome if it is being taken when the child develops either influenza or varicella infection. All three forms of valproate hepatotoxicity have features of mitochondrial injury, and liver histology usually demonstrates microvesicular steatosis with variable amounts of inflammation and cholestasis. Young age ( Likelihood score: A (well known cause of several forms of clinically apparent liver injury). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation Valproic acid levels in breastmilk are low and infant serum levels range from undetectable to low. Breastfeeding during valproic acid monotherapy does not appear to adversely affect infant growth or development; however, and breastfed infants had higher IQs and enhanced verbal abilities than nonbreastfed infants at 6 years of age in one study. A safety scoring system finds valproic acid possible to use during breastfeeding, and a computer model predicted a relatively low infant exposure, consistent with literature reports. If valproic acid is required by the mother, it is not a reason to discontinue breastfeeding. No definite adverse reactions to valproic acid in breastfed infants have been reported. Theoretically, breastfed infants are at risk for valproic acid-induced hepatotoxicity, so infants should be monitored for jaundice and other signs of liver damage during maternal therapy. A questionable case of thrombocytopenia has been reported, so monitor the infant for unusual bruising or bleeding. A rare case of infant baldness might have been caused by valproate in milk. Observe the infant for jaundice and unusual bruising or bleeding. Combination therapy with sedating anticonvulsants or psychotropics may result in infant sedation or withdrawal reactions. ◉ Effects in Breastfed Infants A mother with epilepsy was taking valproic acid 2.4 grams daily and primidone 250 mg 3 times daily during pregnancy and postpartum. During the second week postpartum, her breastfed infant was sedated. Breastfeeding was stopped and the drowsiness cleared. The sedation was possibly caused by primidone in breastmilk although valproic acid might have contributed by increasing primidone levels. Petechiae, thrombocytopenia, anemia, and mild hematuria occurred in a 2.5-month-old breastfed infant whose mother was taking valproic acid 600 mg twice daily. Blood hemoglobin and reticulocytes normalized between 12 and 19 days after discontinuing breastfeeding. The petechiae resolved 35 days after discontinuing breastfeeding and the infant's platelet count had almost reached the normal range by this time. By day 83, the infant's platelet count was well within the normal range. The authors believed the adverse effect to be caused by valproic acid in breastmilk. However, other authors believe that these symptoms were more likely caused by idiopathic thrombocytopenic purpura following a viral infection. Two breastfed infants aged 1 and 3 months whose mothers were taking valproic acid monotherapy 750 and 500 mg daily developed normally and had no abnormal laboratory values. Their plasma levels were 6% and 1.5% or their mother's serum levels, respectively. Six breastfed infants whose mothers were taking valproic acid 750 or 1000 mg daily had no adverse reactions to valproic acid in breastmilk. An exclusively breastfed infants whose mother was taking valproate 1.8 g, topiramate 300 mg, and levetiracetam 2 g, daily during pregnancy and lactation appeared healthy to the investigators throughout the 6- to 8-week study period. In a long-term study on infants exposed to anticonvulsants during breastfeeding, no difference in average intelligence quotient at 3 years of age was found between infants who were breastfed (n = 11) a median of 6 months and those not breastfed (n = 24) when their mothers were taking valproate monotherapy. At 6 years of age, extensive psychological and intelligence testing found that the breastfed infants had higher IQ values than the nonbreastfed infants. A prospective cohort study in Norway followed infants of mothers who took antiepileptic drugs during pregnancy and lactation and compared them to infants of mothers with untreated epilepsy and infants with fathers who took antiepileptics as control groups. Of the 223 mothers studied, 27 were taking valproate monotherapy. Infants were assessed at 6, 18 and 36 months of age. Continuous breastfeeding in children of women using antiepileptic drugs was associated with no greater impaired development than those with no breastfeeding or breastfeeding for less than 6 months. A woman with bipolar disorder who delivered twins and was taking sodium valproate in a therapeutic dosage was started on quetiapine 200 mg and olanzapine 15 mg at 11 pm daily after 20 days postpartum. She withheld breastfeeding during the night and discarded milk pumped at 7 am. She then breastfed her infants until 11 pm. The mother continued feeding the infants on this schedule for 15 months. Monthly follow-up of the infants indicated normal growth and neither the pediatricians nor the parents noted any adverse effects in the infants. The 4-month-old breastfed infant of a mother taking divalproex for bipolar disorder developed patchy hair loss. The extent of nursing and dosage of divalproex were not stated. Divalproex was discontinued and 2 months later, the infant’s hair was normal. The hair loss was possibly caused by valproate. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
||
| 参考文献 | |||
| 其他信息 |
Valproate (Valproic acid) can cause developmental toxicity according to an independent committee of scientific and health experts.
Sodium valproate is the sodium salt of valproic acid. It has a role as a geroprotector. It contains a valproate. Valproate or valproic acid is a branched chain organic acid that is used as therapy of epilepsy, bipolar disorders and migraine headaches and is a well known cause of several distinctive forms of acute and chronic liver injury. Valproate Sodium is the sodium salt form of valproic acid with anti-epileptic activity. Valproate sodium is converted into its active form, valproate ion, in blood. Although the mechanism of action remains to be elucidated, valproate sodium increases concentrations of gamma-aminobutyric acid (GABA) in the brain, probably due to inhibition of the enzymes responsible for the catabolism of GABA. This potentiates the synaptic actions of GABA. Valproate sodium may also affect potassium channels, thereby creating a direct membrane-stabilizing effect. A fatty acid with anticonvulsant and anti-manic properties that is used in the treatment of EPILEPSY and BIPOLAR DISORDER. The mechanisms of its therapeutic actions are not well understood. It may act by increasing GAMMA-AMINOBUTYRIC ACID levels in the brain or by altering the properties of VOLTAGE-GATED SODIUM CHANNELS. See also: Valproic Acid (has active moiety). |
| 分子式 |
C8H15NAO2
|
|
|---|---|---|
| 分子量 |
166.19
|
|
| 精确质量 |
166.096
|
|
| 元素分析 |
C, 57.82; H, 9.10; Na, 13.83; O, 19.25
|
|
| CAS号 |
1069-66-5
|
|
| 相关CAS号 |
99-66-1 (free acid); 1069-66-5 (sodium); 33433-82-8 (calcium); Valproic acid-d4;87745-17-3;Valproic acid-d6;87745-18-4;Valproic acid-d15;362049-65-8;Valproic acid (sodium)(2:1);76584-70-8;Valproic acid-d4 sodium;Valproic acid-d4-1;345909-03-7
|
|
| PubChem CID |
16760703
|
|
| 外观&性状 |
White to off-white crystalline powder
|
|
| 密度 |
1.0803 g/cm3
|
|
| 沸点 |
220ºC at 760 mmHg
|
|
| 熔点 |
300 °C
|
|
| 闪点 |
STABILITY
|
|
| 蒸汽压 |
0.0435mmHg at 25°C
|
|
| LogP |
0.952
|
|
| tPSA |
40.13
|
|
| 氢键供体(HBD)数目 |
0
|
|
| 氢键受体(HBA)数目 |
2
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
11
|
|
| 分子复杂度/Complexity |
98.3
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
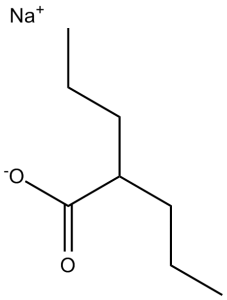
[Na+].[O-]C(C([H])(C([H])([H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])[H])=O
|
|
| InChi Key |
AEQFSUDEHCCHBT-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/C8H16O2.Na/c1-3-5-7(6-4-2)8(9)10;/h7H,3-6H2,1-2H3,(H,9,10);/q;+1/p-1
|
|
| 化学名 |
sodium;2-propylpentanoate
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 100 mg/mL (601.72 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
配方 2 中的溶解度: 5% DMSO 1 95% Corn oil: 1.65mg/ml (9.93mM) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 6.0172 mL | 30.0860 mL | 60.1721 mL | |
| 5 mM | 1.2034 mL | 6.0172 mL | 12.0344 mL | |
| 10 mM | 0.6017 mL | 3.0086 mL | 6.0172 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00431522 | Completed | Drug: Valproic acid, sodium salt |
Bipolar Disorder | Sanofi | December 2004 | Phase 4 |
| NCT05017454 | Completed | Drug: the optimized sodium valproate-loaded nanospanlastic dispersion Drug: mometasone furoate lotion |
Alopecia Areata | Kasr El Aini Hospital | May 1, 2021 | Early Phase 1 |
| NCT04531592 | Withdrawn | Drug: Valproic acid Drug: Isotonic saline solution |
Acute Kidney Injury Ischemia Reperfusion Injury |
Westat | January 2022 | Phase 2 |
| NCT04531579 | Withdrawn | Drug: Isotonic saline solution | Ischemia Reperfusion Injury Acute Kidney Injury |
Westat | January 2022 | Phase 2 |