| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 靶点 |
HDAC1 ( IC50 = 400 μM ); HDAC1 ( IC50 = 0.5-2 mM ); HDAC2; Autophagy; Mitophagy
|
|---|---|
| 体外研究 (In Vitro) |
丙戊酸 (VPA)(0–15 mM;24 和 72 小时)以剂量和时间依赖性方式抑制 Hela 细胞的增殖[1]。丙戊酸(10 mM;24 小时)显着降低细胞核、细胞质和总 HDAC 的活性[1]。当丙戊酸(0-15 mM;24 小时)在 10 mM 产生 G1/M 期停滞和在 1-3 mM 产生 G1 期停滞时,HeLa 细胞中亚 G1 细胞的百分比上升。坏死、细胞凋亡和乳酸脱氢酶 (LDH) 的释放是丙戊酸的其他作用[1]。锂与丙戊酸(0–20 mM;24 小时)协同作用,刺激 Tcf/Lef 依赖性转录[2]。丙戊酸可提高 Neuro2A 细胞的 β-连环蛋白水平(0–5 mM;0–18 小时)[2]。丙戊酸(0-2 mM;0-24 小时)会刺激肝细胞 AMPK 和 ACC 磷酸化[5]。两天内,丙戊酸 (0 -10 mM) 抑制 SCLC 细胞中 NE 肿瘤标志物的生成,同时诱导 Notch1 信号传导和形态分化[6]。
|
| 体内研究 (In Vivo) |
在移植 Kasumi-1 细胞的小鼠中,丙戊酸 (VPA)(500 mg/kg;腹腔注射;每天一次,持续 12 天)可抑制肿瘤血管生成[3]。服用丙戊酸(350 mg/kg;腹腔注射;一次)的大鼠表现出社会行为的改善[4]。在肥胖小鼠中,丙戊酸(0.26% w/v;通过饮用水口服;14 天)可降低血糖、肝脂肪形成和肝脏质量,且不会引起肝毒性[5]。
|
| 酶活实验 |
丙戊酸被广泛用于治疗癫痫和双相情感障碍,也是一种强效致畸物,但其在这些情况下的作用机制尚不清楚。我们报道丙戊酸激活wnt依赖性基因表达,类似于锂,是治疗双相情感障碍的主要药物。然而,丙戊酸通过一种不同的途径起作用,包括直接抑制组蛋白去乙酰化酶(IC(50), HDAC1 = 0.4 mm)。在治疗水平上,丙戊酸模拟组蛋白去乙酰化酶抑制剂曲古霉素A,在培养细胞中引起组蛋白超乙酰化。丙戊酸和曲古抑素A一样,也能激活多种外源性和内源性启动子的转录。此外,丙戊酸和trichostatin A在脊椎动物胚胎中具有非常相似的致畸作用,而丙戊酸的非致畸类似物不会抑制组蛋白去乙酰化酶,也不会激活转录。基于这些观察,我们提出组蛋白去乙酰化酶的抑制为丙戊酸诱导的出生缺陷提供了一种机制,也可以解释丙戊酸治疗双相情感障碍的疗效。[2]
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: HeLa 细胞 测试浓度: 0、1、3、5、10 和 15 mM <孵化持续时间: 24 和 72 小时 实验结果: HeLa 细胞生长呈剂量和时间依赖性减弱,24 和 72 时的 IC50 分别约为 10 和 4 mM H。 蛋白质印迹分析[1][2][5] 细胞类型: HeLa 细胞、Neuro2A 细胞或原代小鼠肝细胞 测试浓度: 10 mM (HeLa); 0、2 和 5 mM(Neuro2A); 0.2、0.4、0.8、1.2 和 2 mM(肝细胞) 孵育时间:24 小时 (HeLa); 0-18 小时(Neuro2A); 0-24小时(肝细胞) 实验结果:乙酰化组蛋白3的形式增加。PARP减少,诱导PARP裂解,并下调Bcl-2。 β-连环蛋白水平增加。增加 AMPK 和 ACC 的磷酸化。 细胞周期分析[1] 细胞类型: HeLa 细胞 测试浓度: 0、1、3、5、10 和15 mM 孵育持续时间: 24 小时 实验结果: 在 1–3 mM 时诱导 G1 期停滞,在 1-3 mM 时显着诱导 G2/M 期停滞10 mM,并在 24 h 时以剂量依赖性方式增加 HeLa 细胞中亚 G1 细胞的百分比。 |
| 动物实验 |
Animal/Disease Models: Female BALB/c nude mice, Kasumi-1 tumor model[3]
Doses: 500 mg/kg Route of Administration: intraperitoneal (ip)injection, daily for 12 days Experimental Results: Inhibited tumor growth and tumor angiogenesis. Inhibited the mRNA and protein expression of VEGF, VEGFR2 and bFGF. Inhibited HDAC activity and increased acetylation of histone H3. Enhanced the accumulation of hyperacetylated histone H3 on VEGF promoters. Animal/Disease Models: Timed-pregnant Long Evans rats[4] Doses: 350 mg/kg Route of Administration: intraperitoneal (ip)injection, once Experimental Results: Demonstrated more social investigation and play fighting than control animals. Animal/Disease Models: Obese phenotype of ob/ob mice[5] Doses: 0.26% (w/v) Route of Administration: Oral via drinking water, 14 days Experimental Results: Revealed a marked reduction in the accumulation of fats in the liver as compared with the untreated mice, Dramatically diminished liver mass to body mass, diminished serum triglyceride concentrations, and did not induce hepatotoxicity. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Absorption The intravenous and oral forms of valproic acid are expected to produce the same AUC, Cmax, and Cmin at steady-state. The oral delayed-release tablet formulation has a Tmax of 4 hours. Differences in absorption rate are expected from other formulations but are not considered to be clinically important in the context of chronic therapy beyond impacting frequency of dosing. Differences in absorption may create earlier Tmax or higher Cmax values on initiation of therapy and may be affected differently by meals. The extended release tablet formulation had Tmax increase from 4 hours to 8 hours when taken with food. In comparison, the sprinkle capsule formulation had Tmax increase from 3.3 hours to 4.8 hours. Bioavailability is reported to be approximately 90% with all oral formulations with enteric-coated forms possibly reaching 100%. Route of Elimination Most drug is eliminated through hepatic metabolism, about 30-50%. The other major contributing pathway is mitochondrial β-oxidation, about 40%. Other oxidative pathways make up an additional 15-20%. Less than 3% is excreted unchanged in the urine. Volume of Distribution 11 L/1.73m2. Clearance 0.56 L/hr/m2 Pediatric patients between 3 months and 10 years of age have 50% higher clearances by weight. Pediatric patients 10 years of age or older approximate adult values. Valproic acid and its salt, sodium valproate, are excreted into human milk in low concentrations. Milk concentrations up to 15% of the corresponding level in the mother's serum have been measured. In two infants, serum levels of valporate were 1.5% and 6.0% of maternal values. Placenta transfer study in non-human primate (NHP) is one of the crucial components in the assessment of developmental toxicity because of the similarity between NHP and humans. To establish the method to determine placenta transfer in non-human primate, toxicokinetics of valproic acid (VPA), a drug used to treat epilepsy in pregnant women, were determined in pregnant cynomolgus monkeys. After mating, pregnancy-proven females were daily administered with VPA at dose levels of 0, 20, 60 and 180 mg/kg by oral route during the organogenesis period from gestation day (GD) 20 to 50. Concentrations of VPA and its metabolite, 4-ene-VPA, in maternal plasma on GDs 20 and 50, and concentrations of VPA and 4-ene-VPA in placenta, amniotic fluid and fetus on GD 50 were analyzed using LC/MS/MS. Following single oral administration of VPA to pregnant monkeys, concentrations of VPA and 4-ene-VPA were generally quantifiable in the plasma from all treatment groups up to 4-24 hours post-dose, demonstrating that VPA was absorbed and the monkeys were systemically exposed to VPA and 4-ene-VPA. After repeated administration of VPA to the monkeys, VPA was detected in amniotic fluid, placenta and fetus from all treatment groups, demonstrating that VPA was transferred via placenta and the fetus was exposed to VPA, and the exposures were increased with increasing dose. Concentrations of 4-ene-VPA in amniotic fluid and fetus were below the limit of quantification, but small amount of 4-ene-VPA was detected in placenta. In conclusion, pregnant monkeys were exposed to VPA and 4-ene-VPA after oral administration of VPA at dose levels of 20, 60 and 180 mg/kg during the organogenesis period. VPA was transferred via placenta and the fetus was exposed to VPA with dose-dependent exposure. The metabolite, 4-ene VPA, was not detected in both amniotic fluid and fetus, but small amount of 4-ene-VPA was detected in placenta. These results demonstrated that proper procedures to investigate placenta transfer in NHP, such as mating and diagnosis of pregnancy via examining gestational sac with ultrasonography, collection of amniotic fluid, placenta and fetus after Caesarean section followed by adequate bioanalysis and toxicokinetic analysis, were established in this study using cynomolugus monkeys. PMID:24278535 View More
Metabolism / Metabolites Most drug is metabolized to glucuronide conjugates (30-50%) of the parent drug or of metabolites. Another large portion is metabolized through mitochondrial β-oxidation (40%). The remainder of metabolism (15-20%) occurs through oxidation, hydroxylation, and dehydrogenation at the ω, ω1, and ω2 positions resulting in the formation of hydroxyls, ketones, carboxyls, a lactone metabolite, double bonds, and combinations. The aim of this study was to investigate the relationship between hepatotoxicity, levels of glucuronide conjugates of valproic acid (VPA), and the toxic metabolites of VPA (4-ene VPA and 2,4-diene VPA). /The study/ also examined whether hepatotoxicity could be predicted by the urinary excretion levels of VPA and its toxic metabolites. VPA was administrated orally in rats in amounts ranging from 20 mg/kg to 500 mg/kg. Free and total (free plus glucuronide conjugated) VPA, 4-ene VPA, and 2,4-diene VPA were quantified in urine and liver using gas chromatography-mass spectrometry. Serum levels of aspartate aminotransferase, alanine aminotransferase, and alpha-glutathione S-transferase (alpha-GST) were also determined to measure the level of hepatotoxicity. The serum alpha-GST level increased slightly at the 20 mg/kg dose, and substantially increased at the 100 and 500 mg/kg dose; aspartate aminotransferase and alanine aminotransferase levels did not change with the administration of increasing doses of VPA. The liver concentration of free 4-ene VPA and the urinary excretion of total 4-ene VPA were the only measures that correlated with the increase in the serum alpha-GST level (p < 0.094 and p < 0.023 respectively). From these results, /it is concluded/ that hepatotoxicity of VPA correlates with liver concentration of 4-ene VPA and can be predicted by the urinary excretion of total 4-ene VPA. PMID:19641884 BACKGROUND AND OBJECTIVE: Sodium valproate is a widely prescribed broad-spectrum antiepileptic drug. It shows high inter-individual variability in pharmacokinetics and pharmacodynamics and has a narrow therapeutic range. /This study/ evaluated the effects of polymorphic uridine diphosphate glucuronosyltransferase (UGT)1A6 (541A>G, 552A>C) metabolizing enzyme on the pharmacokinetics of sodium valproate in the patients with epilepsy who showed toxicity to therapy. METHODS: Genotype analysis of the patients was made with polymerase chain-restriction fragment length polymorphism (RFLP) with sequencing. Plasma drug concentrations were measured with reversed phase high-performance liquid chromatography (HPLC) and concentration-time data were analyzed by using a non-compartmental approach. RESULTS: The results of this study suggested a significant genotypic as well as allelic association with valproic acid toxicity for UGT1A6 (541A>G) or UGT1A6 (552A>C) polymorphic enzymes. The elimination half-life (t 1/2 = 40.2 hr) of valproic acid was longer and the clearance rate (CL = 917 mL/hr) was lower in the poor metabolizers group of UGT1A6 (552A>C) polymorphism who showed toxicity than in the intermediate metabolizers group (t 1/2= 35.5 hr, CL = 1,022 mL/hr) or the extensive metabolizers group (t 1/2= 25.4 hr, CL = 1,404 mL/hr). CONCLUSION: /These/ findings suggest that the UGT1A6 (552A>C) genetic polymorphism plays a significant role in the steady state concentration of valproic acid, and it thereby has an impact on the toxicity of the valproic acid used in the patients with epilepsy. PMID:23749495 Biotransformation /of valproic acid/ is primarily hepatic. Some metabolites may have pharmacologic or toxic activity. Rate of metabolism is faster in children and in patients concurrently using enzyme-inducing medications, such as phenytoin, phenobarbital, primidone, and carbamazepine. Valproate is metabolized almost entirely by the liver. In adult patients on monotherapy, 30- 50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial -oxidation is the other major metabolic pathway, typically accounting for over 40% of the dose. Usually, less than 15-20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine. Valproic acid has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-(2-propylpentanoyloxy)oxane-2-carboxylic acid, 4-Hydroxyvalproate, 3-Hydroxyvalproate, 5-Hydroxyvalproate, and 4-ene-valproate. Valproic acid is rapidly absorbed from gastrointestinal tract. Valproic acid is metabolized almost entirely by the liver. In adult patients on monotherapy, 30-50% of an administered dose appears in urine as a glucuronide conjugate. Mitochondrial oxidation is the other major metabolic pathway, typically accounting for over 40% of the dose. These products include 2-n-propylpent-2-enoic acid (delta 2,3 VPE) and several coenzyme A (CoA) derivatives including VPA-CoA, and delta 2,3 VPE-CoA. Usually, less than 15-20% of the dose is eliminated by other oxidative mechanisms. Less than 3% of an administered dose is excreted unchanged in urine (A308). Half Life: 9-16 hours (following oral administration of 250 mg to 1000 mg). Biological Half-Life 13-19 hours. The half-life in neonates ranges from 10-67 hours while the half-life in pediatric patients under 2 months of age ranges from 7-13 hours. In children the half-life of valproic acid alone is 10 to 11 hours; when other medications are added, half-life may be reduced to 8 to 9 hours. Half-lives of up to 30 hours have been reported in overdosage. International Programme on Chemical Safety (IPCS); Poisons Information Monograph: Valproic Acid (PIM 551) (1997) Available from, as of May 30, 2007: https://www.inchem.org/pages/pims.html Variable, from 6 to 16 hours; may be considerably longer in patients with hepatic function impairment, in the elderly, and in children up to 18 months of age; may be considerably shorter in patients receiving hepatic enzyme-inducing anticonvulsants. In one study, the half life in children under 10 days ranged from 10 to 67 hours compared to a range of 7 to 13 hours in children greater than 2 months. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Very little information is available on the clinical use of divalproex during breastfeeding. However, divalproex is rapidly metabolized in the body to the active drug valproic acid. Valproic acid levels in breastmilk are low and infant serum levels range from undetectable to low. Breastfeeding during valproic acid monotherapy does not appear to adversely affect infant growth or development, and breastfed infants had higher IQs and enhanced verbal abilities than nonbreastfed infants at 6 years of age in one study. A safety scoring system finds valproic acid possible to use during breastfeeding. If valproic acid is required by the mother, it is not necessarily a reason to discontinue breastfeeding. No definite adverse reactions to valproic acid in breastfed infants have been reported. Theoretically, breastfed infants are at risk for valproic acid-induced hepatotoxicity, so infants should be monitored for jaundice and other signs of liver damage during maternal therapy. A questionable case of thrombocytopenia has been reported, so monitor the infant for unusual bruising or bleeding. A rare case of infant baldness might have been caused by valproate in milk. Observe the infant for jaundice and unusual bruising or bleeding. Combination therapy with sedating anticonvulsants or psychotropics may result in infant sedation or withdrawal reactions. ◉ Effects in Breastfed Infants A mother with epilepsy was taking valproic acid 2.4 grams daily and primidone 250 mg 3 times daily during pregnancy and postpartum. During the second week postpartum, her breastfed infant was sedated. Breastfeeding was stopped and the drowsiness cleared. The sedation was possibly caused by primidone in breastmilk although valproic acid might have contributed by increasing primidone levels. Petechiae, thrombocytopenia, anemia, and mild hematuria occurred in a 2.5-month-old breastfed infant whose mother was taking valproic acid 600 mg twice daily. Blood hemoglobin and reticulocytes normalized between 12 and 19 days after discontinuing breastfeeding. The petechiae resolved 35 days after discontinuing breastfeeding and the infant's platelet count had almost reached the normal range by this time. By day 83, the infant's platelet count was well within the normal range. The authors believed the adverse effect to be caused by valproic acid in breastmilk. However, other authors believe that these symptoms were more likely caused by idiopathic thrombocytopenic purpura following a viral infection. Two breastfed infants aged 1 and 3 months whose mothers were taking valproic acid monotherapy 750 and 500 mg daily developed normally and had no abnormal laboratory values. Their plasma levels were 6% and 1.5% or their mother's serum levels, respectively. Six breastfed infants whose mothers were taking valproic acid 750 or 1000 mg daily had no adverse reactions to valproic acid in breastmilk. An exclusively breastfed infants whose mother was taking valproate 1.8 g, topiramate 300 mg, and levetiracetam 2 g, daily during pregnancy and lactation appeared healthy to the investigators throughout the 6- to 8-week study period. In a long-term study on infants exposed to anticonvulsants during breastfeeding, no difference in average intelligence quotient at 3 years of age was found between infants who were breastfed (n = 11) a median of 6 months and those not breastfed (n = 24) when their mothers were taking valproate monotherapy. At 6 years of age, extensive psychological and intelligence testing found that the breastfed infants had higher IQ values than the nonbreastfed infants. A prospective cohort study in Norway followed infants of mothers who took antiepileptic drugs during pregnancy and lactation and compared them to infants of mothers with untreated epilepsy and infants with fathers who took antiepileptics as control groups. Of the 223 mothers studied, 27 were taking valproate monotherapy. Infants were assessed at 6, 18 and 36 months of age. Continuous breastfeeding in children of women using antiepileptic drugs was associated with no greater impaired development than those with no breastfeeding or breastfeeding for less than 6 months. A woman with bipolar disorder who delivered twins and was taking sodium valproate in a therapeutic dosage was started on quetiapine 200 mg and olanzapine 15 mg at 11 pm daily after 20 days postpartum. She withheld breastfeeding during the night and discarded milk pumped at 7 am. She then breastfed her infants until 11 pm. The mother continued feeding the infants on this schedule for 15 months. Monthly follow-up of the infants indicated normal growth and neither the pediatricians nor the parents noted any adverse effects in the infants. The 4-month-old breastfed infant of a mother taking divalproex for bipolar disorder developed patchy hair loss. The extent of nursing and dosage of divalproex were not stated. Divalproex was discontinued and 2 months later, the infant’s hair was normal. The hair loss was possibly caused by valproate. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. |
| 参考文献 |
|
| 其他信息 |
Valproate semisodium is a mixture of valproic acid and its sodium salt in a 1:1 molar ratio. It is used for the management and treatment of seizure disorders, mania, and prophylactic treatment of migraine headache. It has a role as an antimanic drug, an anticonvulsant and a GABA agent. It contains a valproic acid and a sodium valproate.
Divalproex Sodium is a stable coordination compound comprised of sodium valproate and valproic acid with anticonvulsant and antiepileptic activities. Divalproex dissociates to the valproate ion in the gastrointestinal tract. This agent binds to and inhibits gamma-aminobutyric acid (GABA) transaminase and its anticonvulsant activity may be exerted by increasing brain concentration of GABA and by inhibiting enzymes that catabolize GABA or block the reuptake of GABA into glia and nerve endings. Divalproex may also work by suppressing repetitive neuronal firing through inhibition of voltage-sensitive sodium channels. A fatty acid with anticonvulsant and anti-manic properties that is used in the treatment of EPILEPSY and BIPOLAR DISORDER. The mechanisms of its therapeutic actions are not well understood. It may act by increasing GAMMA-AMINOBUTYRIC ACID levels in the brain or by altering the properties of VOLTAGE-GATED SODIUM CHANNELS. See also: Valproic Acid (has active moiety). |
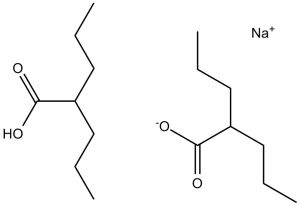
| 分子式 |
C8H16O2.C8H15O2.NA
|
|
|---|---|---|
| 分子量 |
310.41
|
|
| 精确质量 |
310.212
|
|
| CAS号 |
76584-70-8
|
|
| 相关CAS号 |
Valproic acid;99-66-1; Valproic acid sodium;1069-66-5;Valproic acid-d4;87745-17-3;Valproic acid-d6;87745-18-4;Valproic acid-d15;362049-65-8;Valproic acid (sodium)(2:1);76584-70-8;Valproic acid-d4 sodium;Valproic acid-d4-1;345909-03-7
|
|
| PubChem CID |
23663956
|
|
| 外观&性状 |
Typically exists as solid at room temperature
|
|
| 沸点 |
220ºC at 760 mmHg
|
|
| 熔点 |
222ºC
|
|
| 闪点 |
116.6ºC
|
|
| LogP |
3.24
|
|
| tPSA |
77.43
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
10
|
|
| 重原子数目 |
21
|
|
| 分子复杂度/Complexity |
192
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
[Na+].[O-]C(C([H])(C([H])([H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])[H])=O.O([H])C(C([H])(C([H])([H])C([H])([H])C([H])([H])[H])C([H])([H])C([H])([H])C([H])([H])[H])=O
|
|
| InChi Key |
MSRILKIQRXUYCT-UHFFFAOYSA-M
|
|
| InChi Code |
InChI=1S/2C8H16O2.Na/c2*1-3-5-7(6-4-2)8(9)10;/h2*7H,3-6H2,1-2H3,(H,9,10);/q;;+1/p-1
|
|
| 化学名 |
sodium;2-propylpentanoate;2-propylpentanoic acid
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.2215 mL | 16.1077 mL | 32.2155 mL | |
| 5 mM | 0.6443 mL | 3.2215 mL | 6.4431 mL | |
| 10 mM | 0.3222 mL | 1.6108 mL | 3.2215 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00431522 | Completed | Drug: Valproic acid, sodium salt |
Bipolar Disorder | Sanofi | December 2004 | Phase 4 |
| NCT05017454 | Completed | Drug: the optimized sodium valproate-loaded nanospanlastic dispersion Drug: mometasone furoate lotion |
Alopecia Areata | Kasr El Aini Hospital | May 1, 2021 | Early Phase 1 |
| NCT04531592 | Withdrawn | Drug: Valproic acid Drug: Isotonic saline solution |
Acute Kidney Injury Ischemia Reperfusion Injury |
Westat | January 2022 | Phase 2 |
| NCT04531579 | Withdrawn | Drug: Isotonic saline solution | Ischemia Reperfusion Injury Acute Kidney Injury |
Westat | January 2022 | Phase 2 |