| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
SRMS (IC50 = 18 nM); ACK1 (IC50 = 19 nM); B-Raf (V600E) (IC50 = 48 nM); MAP4K5 (KHS1) (IC50 = 51 nM); C-Raf (IC50 = 48 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Vemurafenib (PLX4032) 特异性抑制 BRAF 突变细胞中的 RAF/MEK/ERK 通路[1]。在 17 种黑色素瘤细胞系中,RG7204 是表达 RAFV600E 但不表达 BRAFWT 的细胞增殖的有效抑制剂。高浓度的维莫非尼 (RG7204) 会导致 CHL-1 细胞中 MEK 和 ERK 磷酸化[2]。对 PLX4032 的耐药性可能是由异位表达的黑色素瘤细胞中 EGFR 的表达引起的[3]。
BRAF(V600E)突变在几种人类癌症中很常见,尤其是黑色素瘤。RG7204(PLX4032)是一种BRAF(V600E)激酶活性的小分子抑制剂,目前正处于II期和III期临床试验中。在这里,我们使用已建立的恶性黑色素瘤体外和体内模型报告了RG7204抗肿瘤活性的临床前特征。RG7204能有效抑制一组肿瘤细胞系的增殖和丝裂原活化蛋白/细胞外信号调节激酶(ERK)激酶和ERK磷酸化,包括表达BRAF(V600E)或密码子600处改变的其他突变BRAF蛋白的黑色素瘤细胞系[2]。 |
| 体内研究 (In Vivo) |
Vemurafenib (PLX4032, 20, 25, 75 mg/kg, po) 以剂量依赖性方式抑制肿瘤生长,较高的暴露量会导致携带 BRAF 突变的异种移植物中的肿瘤消退[1]。在携带 LOX 肿瘤异种移植物的小鼠中,RG7204(12.5、25 和 75 mg/kg,口服)显着抑制肿瘤生长并导致肿瘤消退[2]。
在表达BRAF(V600E)的黑色素瘤的几种肿瘤异种移植物模型中,研究人员发现,RG7204治疗以剂量依赖的方式导致部分或完全的肿瘤消退,并提高了动物存活率。在所测试的任何体内模型中,任何剂量组均未观察到毒性。 |
| 酶活实验 |
PLX4032激酶选择性如文中所述,当激酶选择性面板扩展到200多个成员时,发现另外几种激酶对PLX4032s敏感。这些激酶中的大多数在较低的ATP浓度下进行了测定(计数器筛选为10μM,而RAF激酶为100μM);因为PLX4032是在较低ATP浓度下的竞争性抑制剂测定,导致较低的IC50值。在PLX4032的150多种化学类似物中,B-RAFV600E的生化效力与针对B-RAF突变细胞的细胞活性之间存在良好的相关性。这种相关性并不取决于对B-RAFV600E和野生型B-RAF的相对效力。因此,我们认为黑色素瘤的疗效主要来自对突变型B-RAV的抑制;未来的研究可能会探索脱靶在其他适应症中的作用[1]。
当PLX4032与B-RAFV600E共结晶时,不对称单元中激酶结构域的两个独特分子采用侧对侧二聚体结构,如之前RAF晶体结构中观察到的那样。以前,PLX4720与野生型B-RAF共结晶,只有部分配体占据(apo)的原聚体采用DFG外构象,代表激酶的失活状态。然而,PLX4032与B-RAFV600E共结构中的载脂蛋白原聚体显示出DFG与激活环的构象,该激活环通过Glu600和Lys507之间的盐桥锁定在ATP结合位点之外(图1D)。随后对与B-RAFV600E共结晶的PLX4720结构的分析表明,载脂蛋白原聚体在构象上显示出DFG,这表明这种性质是由突变决定的。有趣的是,推测apo原聚体的构象可能决定了正文中描述的反常激活。晶体结构捕获的构象差异(图1C)表明,尽管野生型B-RAF在活性(DFG-in)和非活性(DFG-out)配置之间处于动态平衡,但致癌BRAF突变如V600E通过将平衡向活性(DFG in)配置转变来诱导组成型激酶活性。我们认为,在构象上选择性结合DFG可能有助于获得较大的安全裕度,因为此类抑制剂会抑制肿瘤生长,但不会影响野生型B-RAF激酶介导的重要生物学功能[1]。 |
| 细胞实验 |
简而言之,将细胞以每孔 1,000 至 5,000 个细胞的密度接种在体积为 180 μL 的 96 孔微量滴定板中。 Vemurafenib (RG7204) 在含有 1% DMSO 的培养基中制备,浓度为最终测定浓度的 10 倍。细胞铺板二十四小时后,将 20 μL 适当的稀释液一式两份添加到板中。细胞铺板六天后,根据程序测试板的增殖情况。
对于细胞系的样品制备,在化合物处理前1天,将细胞以适当的密度(70-75%融合)接种在六孔板中。在37°C下以不同药物浓度进行化合物处理2小时后,立即收获细胞并裂解。对于肿瘤异种移植物的样品制备,在指定的时间点收获肿瘤并储存在-80°C下。在2-5mL裂解缓冲液存在下通过均质化提取蛋白质。在冰上孵育20至30分钟后,将裂解物以14000rpm离心15分钟。测定裂解物的蛋白质浓度。 细胞裂解物和肿瘤裂解物的等量总蛋白在4%至12%的NuPage梯度聚丙烯酰胺凝胶上溶解,并用指定的抗体印迹。化学发光信号由增强化学发光加蛋白质印迹检测试剂产生,并用Fujifilm LAS-3000成像仪检测。使用Multi-Gauge软件确定特定条带的密度定量。[2] |
| 动物实验 |
Athymic nude mice have a lifespan of 13 to 14 weeks and weigh between 23 and 25 g. 2×106 cells in 0.2 mL of PBS are injected subcutaneously into the right lateral flank for the LOX xenografts. In an aqueous vehicle containing 2% Klucel LF and pH 4-adjusted with diluted HCl, vemurafenib (RG7204), formulated as MBP, is suspended at the required concentration as needed for each dose group. There are 250-mg capsules of NSC 362856. Opened capsules are collected into a single bulk supply. NSC 362856 is first dissolved in 100% DMSO, then the DMSO is diluted with saline to create a final milky white suspension in 10% DMSO/90% saline (pH 3.4), which is the stock dosing material.
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Vemurafenib is well absorbed after oral administration. Peak concentrations are reached in 3 hours when an oral dose of 960 mg twice daily for 15 days has been given to patients. In the same conditions, Vemurafenib presents a Cmax of 62 mcg/ml and AUC of 601 mcg h/ml. It is unknown how food affects the absorption of vemurafenib. It presents an accumulation ratio of 7.36 after repeating doses of 960 mg Analysis showed that 94% of administered Vemurafenib is excreted via feces and 1% is excreted by urine. The estimation of the volume of distribution for Vemurafenib is 106 L. The total body clearance is 31 L/day. Following oral administration of (14)C-vemurafenib 960 mg in the tablet formulation, plasma samples were analyzed over 48 hours for vemurafenib and its metabolites. Mean data showed that vemurafenib and its metabolites represented 95% and 5% of the components in plasma, respectively. Vemurafenib is highly bound (> 99%) to human albumin and alpha-1 acid glycoprotein plasma proteins. The population apparent volume of distribution for vemurafenib in metastatic melanoma patients is estimated to be 106 L (with 66% inter-patient variability). The bioavailability of vemurafenib has not been determined. Following oral administration of vemurafenib at 960 mg twice daily for 15 days to patients with metastatic melanoma, the median Tmax was approximately 3 hours. Following 15 days of dosing at 960 mg twice daily, the mean (+ or - SD) Cmax and AUC0-12 were 62 ug/mL + or - 17 and 601 + or - 170 ug*h/mL, respectively. The median accumulation ratio estimate from the population pharmacokinetic analysis for the twice daily regimen is 7.36, with steady state achieved at approximately 15 to 22 days following dosing at 960 mg twice daily. At steady state, the mean vemurafenib exposure in plasma is stable (concentrations before and 2-4 hours after the morning dose) as indicated by the mean ratio of 1.13. The potential effect of food on vemurafenib absorption has not been studied. In clinical trials, vemurafenib was administered without regard to food. Following oral administration of (14)C-vemurafenib 960 mg in the tablet formulation, approximately 94% of the radioactive dose was recovered in feces and approximately 1% was recovered in the urine. The population apparent clearance of vemurafenib in patients with metastatic melanoma is estimated to be 31 L/day (with 32% inter-patient variability). For more Absorption, Distribution and Excretion (Complete) data for Vemurafenib (6 total), please visit the HSDB record page. Metabolism / Metabolites Vemurafenib is metabolized by CYP3A4 and the metabolites make up 5% of the components in plasma. The parent compound makes up for the remaining 95%. The results from in vitro studies indicate that CYP3A4 was the major enzyme responsible in the metabolism of vemurafenib. The formation of mono-hydroxyl metabolites were inhibited for approximately 82% using the CYP inhibitor ketoconazole. No significant inhibition in the metabolism was observed in human liver microsomes in the presence of quinidine (CYP2D6 inhibitor), sulfaphenazole (CYP2C9 inhibitor), tranylcypromine (CYP2A6 inhibitor) and (-)-N-3-benzyl-phenobarbital (CYP2C19 inhibitor). In addition, CYP3A4 was responsible for the formation of the mono-hydroxylation metabolites. In vitro metabolism was analyzed for rat, mouse, dog, cynomolgus and human. The metabolism of vemurafenib was investigated both in vitro using microsomes and hepatocytes of various species and in vivo in rat, dog and human. In vitro analysis of vemurafenib metabolism in liver hepatocytes at the concentration of 10 uM, humans, dogs, and cynomolgus monkeys did not metabolize vemurafenib extensively (unchanged vemurafenib > or = 89%). In study /of patients/, identification of vemurafenib and metabolites in plasma, feces and urine was made for the first 96 hr, with a total collection period of 432 hrs (18 days). Mean data from the 7 patients indicated that over the period investigated (0 to 96 hours), potential metabolites each accounted for < 0.5% of the total administered dose in urine and .6% of the total administered dose in feces. In pooled fecal samples up to 48 hours post post-dose, parent compound accounted for at least 94% of total radioactivity (37% of the dose). In fecal samples taken 48-96 hr post-dose, the amount of metabolites increased, with M6, M3, and M8 representing approximately 19%, 14% and 12%, of the total chromatographic peak area, respectively (mean values) or 3%, 5% and 4% of the dose, respectively. Over the 0-96 hr collection period, potential metabolites M3 (mono-hydroxy) and M6 (glucosylation) each accounted for <0.5% of the total administered dose in urine. Vemurafenib accounted for approximately 1% of the total dose in urine. Biological Half-Life The elimination half-life of Vemurafenib is estimated to be 57 hours (range of 30-120 hours). Single dose studies to determine pharmacokinetics were conducted in mouse, rat, rabbit, dog and monkey. In all pre-clinical species, half-lifes were between 2 and 5 hours ... . Only after intraperitoneal (IP) administration in mice, the half-life was much longer (20.6 h). Compared with other species, rabbits showed higher plasma exposure levels with a longer mean terminal half-life between 12 and 18 hours. The median of the individual elimination half-life estimates for vemurafenib is 57 hours (the 5th and 95th percentile range is 30 to 120 hours). |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
In large clinical trials of vemurafenib, abnormalities in routine liver tests were common and serum aminotransferase elevations occurred in up to one third of patients. ALT and AST values greater than 5 times the upper limit of normal (ULN) occurred in 3% of patients, and rare instances of clinically apparent liver injury were reported, but the clinical features of the injury have not been described. The onset of liver test abnormalities was typically within 3 to 6 weeks of starting vemurafenib, and the abnormalities resolved rapidly either spontaneously or with temporary drug discontinuation. Vermurafenib has also been linked to instances of drug related rash with eosinophilia and systemic manifestations (DRESS) as well as Stevens Johnson syndrome, both of which can be accompanied by liver dysfunction and in some cases jaundice with clinically apparent liver injury. Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury. Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the clinical use of vemurafenib during breastfeeding. Because vemurafenib is more than 99% bound to plasma proteins, the amount in milk is likely to be low. However, its half-life is 57 hours and it might accumulate in the infant. The manufacturer recommends that breastfeeding be discontinued during vemurafenib therapy and for 2 weeks after the final dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding Vemurafenib highly binds to plasma proteins where >99% of the administered dose will be found protein bound to serum albumin and alpha-1 acid glycoprotein. Interactions Concomitant use of vemurafenib with drugs known to prolong the QT interval, including class Ia (e.g., quinidine, procainamide) and class III (e.g., amiodarone, sotalol) antiarrhythmic agents, some antipsychotic agents (e.g., chlorpromazine, thioridazine, haloperidol, asenapine, olanzapine, paliperidone, pimozide, quetiapine, ziprasidone), some antibiotics (e.g., gatifloxacin, moxifloxacin), and tetrabenazine is not recommended by the manufacturer. Concomitant use of vemurafenib with CYP2C9 substrates may result in increased plasma concentrations of the CYP2C9 substrate and possible toxicity. When the CYP2C9 substrate warfarin was administered concomitantly with vemurafenib, the systemic exposure of S-warfarin increased by 18%. Vemurafenib and warfarin should be used concomitantly with caution and additional monitoring of the international normalized ratio (INR) should be considered. Concomitant use of vemurafenib with CYP3A4 substrates may result in decreased plasma concentrations of the CYP3A4 substrate and possible decreased efficacy. When the CYP3A4 substrate midazolam was administered concomitantly with vemurafenib, the systemic exposure of midazolam decreased by 39%. Concomitant use of vemurafenib with CYP3A4 substrates that have a narrow therapeutic index should be avoided. Concomitant use of vemurafenib with CYP2D6 substrates may result in increased plasma concentrations of the CYP2D6 substrate and possible toxicity. When the CYP2D6 substrate dextromethorphan was administered concomitantly with vemurafenib, the systemic exposure of dextromethorphan increased by 47%. Concomitant use of vemurafenib with CYP2D6 substrates that have a narrow therapeutic index should be avoided. If concomitant use cannot be avoided, dosage reduction of the CYP2D6 substrate should be considered, and the drugs should be used concomitantly with caution. For more Interactions (Complete) data for Vemurafenib (9 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Vemurafenib is used for the treatment of unresectable or metastatic melanoma with BRAF V600E mutation. Vemurafenib is designated an orphan drug by the US Food and Drug Administration (FDA) for the treatment of this cancer. An FDA-approved diagnostic test (e.g., cobas 4800 BRAF V600 Mutation Test) is required to confirm the presence of the BRAF V600E mutation prior to initiation of therapy. /Included in US product label/ Zelboraf is not recommended for use in patients with wild-type BRAF melanoma. Drug Warnings Serious hypersensitivity reactions (e.g., anaphylaxis, generalized rash and erythema, hypotension) have been reported in patients receiving vemurafenib. Vemurafenib should be permanently discontinued in patients who experience a severe hypersensitivity reaction. Photosensitivity reactions (mild to severe) have been reported in 33-49% of patients receiving vemurafenib in clinical trials. If intolerable grade 2 (i.e., tender erythema covering 10-30% of body surface area) or greater reaction occurs, the dosage of vemurafenib should be reduced. Vemurafenib prolongs the QT interval in a concentration-dependent manner. In a multicenter, open-label, phase 2 study, QT interval prolongation was evaluated in patients with BRAF V600E mutation-positive, metastatic melanoma who were receiving vemurafenib (960 mg twice daily). A maximum mean corrected QT (QTc) interval change from baseline of 12.8 msec during the first month of treatment and 15.1 msec during the first 6 months of treatment was observed in these patients. The manufacturer does not recommend initiation of vemurafenib in patients with electrolyte abnormalities unresponsive to corrective measures or congenital long QT syndrome. In addition, concomitant use of vemurafenib with drugs known to prolong the QT interval (e.g., class Ia and III antiarrhythmic agents) is not recommended. ECGs and serum electrolyte concentrations, including concentrations of potassium, magnesium, and calcium, should be obtained prior to initiation of therapy or following dosage modification, and monitored 15 days following initiation of therapy, then monthly for the first 3 months of treatment, and then every 3 months thereafter or more often as clinically indicated. Interruption or discontinuance of vemurafenib may be necessary if increases in the QTc interval occur during therapy with the drug. Severe skin reactions (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis) have been reported with vemurafenib. If severe skin reactions occur, vemurafenib therapy should be permanently discontinued. For more Drug Warnings (Complete) data for Vemurafenib (18 total), please visit the HSDB record page. Pharmacodynamics BRAF activation results in cell growth, proliferation, and metastasis. BRAF is an intermediary molecule in MAPK whose activation depends on ERK activation, elevation of cyclin D1 and cellular proliferation. The mutation V600E produces a constitutively form of BRAF. Vemurafenib has been shown to reduce all activation markers related to BRAF; in clinical trials, vemurafenib treatment showed a reduction of cytoplasmic phosphorylated ERK and a cell proliferation driven by Ki-67. Studies also reported decrease in MAPK-related metabolic activity. All the different reports indicate thet Vemurafenib generates an almost complete inhibition of the MAPK pathway. |
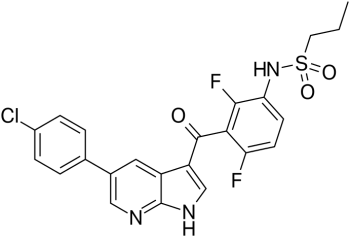
| 分子式 |
C23H18CLF2N3O3S
|
|---|---|
| 分子量 |
489.92
|
| 精确质量 |
489.072
|
| 元素分析 |
C, 56.39; H, 3.70; Cl, 7.24; F, 7.76; N, 8.58; O, 9.80; S, 6.54
|
| CAS号 |
918504-65-1
|
| 相关CAS号 |
Vemurafenib-d5;1365986-90-8;Vemurafenib-d7;1365986-73-7; 918505-61-0 (analog); 918504-65-1
|
| PubChem CID |
42611257
|
| 外观&性状 |
White to off-white crystalline solid
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
711.4±70.0 °C at 760 mmHg
|
| 熔点 |
260-262 °C
|
| 闪点 |
384.0±35.7 °C
|
| 蒸汽压 |
0.0±2.3 mmHg at 25°C
|
| 折射率 |
1.653
|
| LogP |
4.26
|
| tPSA |
100.3
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
790
|
| 定义原子立体中心数目 |
0
|
| SMILES |
O=C(C1C(F)=C(NS(CCC)(=O)=O)C=CC=1F)C1C2C(=NC=C(C3C=CC(Cl)=CC=3)C=2)NC=1
|
| InChi Key |
GPXBXXGIAQBQNI-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C23H18ClF2N3O3S/c1-2-9-33(31,32)29-19-8-7-18(25)20(21(19)26)22(30)17-12-28-23-16(17)10-14(11-27-23)13-3-5-15(24)6-4-13/h3-8,10-12,29H,2,9H2,1H3,(H,27,28)
|
| 化学名 |
N-[3-[5-(4-chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl]-2,4-difluorophenyl]propane-1-sulfonamide
|
| 别名 |
Vemurafenib; RO5185426; RG7204; PLX 4032; RG 7204; RO 5185426; RG-7204; RO5185426; PLX4032; PLX-4032; trade name: Zelboraf; N-(3-(5-(4-Chlorophenyl)-1H-pyrrolo[2,3-b]pyridine-3-carbonyl)-2,4-difluorophenyl)propane-1-sulfonamide;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (4.25 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (4.25 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: 4% DMSO +30% PEG 300 +5% Tween 80 +ddH2O: 5mg/mL 配方 4 中的溶解度: 3.33 mg/mL (6.80 mM) in 1.5% CMC-Na/saline water (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0411 mL | 10.2057 mL | 20.4115 mL | |
| 5 mM | 0.4082 mL | 2.0411 mL | 4.0823 mL | |
| 10 mM | 0.2041 mL | 1.0206 mL | 2.0411 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study in Patients Previously Enrolled in a Genentech and/or F. Hoffmann-La Roche Ltd Sponsored Atezolizumab Study
CTID: NCT03768063
Phase: Phase 3 Status: Recruiting
Date: 2024-11-20
Mol Cancer Ther; 15(8); 1859–69, 2016 |
 |
 |