| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 2mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Calcium channel; Permeability-glycoprotein (P-gp); CYP3A4[1]
|
|---|---|
| 体外研究 (In Vitro) |
EverFluor FL Verapamil (EFV)以浓度依赖性方式抑制 TR-iBRB2 细胞,而 Verapamil 以浓度抑制方式抑制 TR-iBRB2 细胞,IC50 为 98.0 μM [4]。
TR-iBRB2细胞摄取EverFluor FL Verapamil (EFV)的功能分析[4] 在TR-iBRB2细胞中研究了EFV摄取的功能,观察到EFV摄取线性增加至少10分钟,初始摄取率为65.3 ± 6.7 μL/(min·mg蛋白质)(图4a)。在4°C时,EFV摄取显著降低了57.8%(图4a),在用Li+或K+替代Na+的缓冲液进行的实验中,没有观察到EFV摄取的显著变化(图4b)。在TR-iBRB2细胞中研究了细胞外和细胞内pH值对EFV摄取的影响。在pH 6.4和8.4时,EFV的摄取与pH 7.4时没有显著差异(图4c),而用NH4Cl对TR-iBRB2细胞进行急性处理后,EFV摄取显著降低了34%(图4d)。 EverFluor FL Verapamil (EFV)对TR-iBRB2细胞摄取的抑制分析[4] 体外分布分析表明,EFV在内外BRB转运。特别是,已知内部BRB可以滋养三分之二的视网膜组织。因此,研究了几种化合物对TR-iBRB2细胞摄取EFV的抑制作用(表I),阳离子药物,包括地昔帕明、丙咪嗪、普萘洛尔和维拉帕米,显著抑制了EFV摄取47%以上。此外,阳离子药物,包括奎尼丁、吡拉明和噻吗洛尔,适度抑制EFV摄取超过25%,而西咪替丁、可乐定、金刚烷胺、乙酰唑胺、胆碱、四乙基铵(TEA)、1-甲基-4-苯基吡啶(MPP+)、左旋肉碱、血清素和对氨基马尿酸(PAH)没有产生显著影响。此外,EFV摄取的抑制分析表明,维拉帕米对EFV摄取具有浓度依赖性抑制作用,IC50为98.0μM(图4e)。 目的:研究维拉帕米在血视网膜屏障(BRB)处的血视网膜转运。 方法:采用EverFluor FL Verapamil (EFV)作为维拉帕米的荧光探针,通过大鼠颈总动脉输注研究其在BRB中的转运。分别用TR-iBRB2细胞和RPE-J细胞研究了EFV在内外BRB的转运。 结果:在细胞不可渗透化合物的弱信号期间,视网膜组织中检测到EverFluor FL Verapamil (EFV)的信号。在TR-iBRB2细胞中,EFV的定位不同于溶酶体运动剂LysoTracker®Red的定位,并且不会因NH4Cl的急性处理而改变。在RPE-J细胞中,部分观察到EFV的点状分布,经NH4Cl急性处理后,这种分布有所减少。TR-iBRB2细胞对EFV的摄取是温度依赖性的,与膜电位和pH无关,NH4Cl处理显著降低了EFV的吸收,而不同的细胞外pH和V-ATP酶抑制剂没有显著影响。TR-iBRB2细胞对EFV的摄取被阳离子药物抑制,维拉帕米以浓度依赖的方式抑制,IC50为98.0μM。 结论:我们的研究结果提供了视觉证据,支持载体介导的维拉帕米在BRB血视网膜转运的意义[4]。 |
| 体内研究 (In Vivo) |
房颤时,维拉帕米(面部)可用于控制房室结反应,避免房室折返性心动过速[2]。将维拉帕米静脉注射到前胸部区域的股静脉中。冠状动脉闭合后 45 分钟内,维拉帕米(1 mg/kg)可显着降低室性心律失常的发生率,例如室性心动过速(VT)、心室颤动(VF)和室性早搏(PVC)。缺血性心脏导致总体心律失常评分显着增加。给予 1 mg/kg 维拉帕米可有效抑制心血管诱发的心律失常总体评分的上升[5]。
维拉帕米的抗心律失常作用是在人们意识到它是一种钙离子拮抗剂之前观察到的。静脉注射Verapamil/维拉帕米在终止阵发性往复式房室心动过速方面非常有效,无论是与预激有关还是仅涉及房室结。它持续减缓和规范房颤患者的心室反应,通常会增加房扑患者的房室结传导阻滞程度,尽管偶尔会导致窦性心律的恢复。口服可用于预防房室折返性心动过速,也可用于调节房颤患者的房室结反应。室性心动过速的有利反应是罕见的,然后在特定的良性品种中可见。维拉帕米是终止阵发性室上性心动过速的首选药物。[2] 干预:患者接受美托洛尔(Seloken ZOC 200 mg o.d.)或Verapamil/维拉帕米(Isotin Retarded 240 b.i.d.)治疗。研究中允许使用乙酰水杨酸、ACE抑制剂、降脂药物和长效硝酸盐。 终点:死亡、非致命性心血管事件,包括急性心肌梗死、致残或不稳定型心绞痛、脑血管或外周血管事件。反映生活质量的心理变量,即心身症状、睡眠障碍和总体生活满意度评估。 结果:美托洛尔和维拉帕米治疗的患者合并心血管事件的发生率分别为30.8%和29.3%,没有差异。美托洛尔和维拉帕米治疗患者的总死亡率分别为5.4%和6.2%。两组的心血管死亡率均为4.7%。美托洛尔和维拉帕米治疗的患者中,非致死性心血管事件的发生率分别为26.1%和24.3%。两个治疗组的心身症状和睡眠障碍都得到了显著改善。变化幅度很小,不同治疗之间没有差异。两种药物的生活满意度都没有变化。因副作用而退出的比例分别为11.1%和14.6%。 结论:这项长期研究表明,这两种药物都具有良好的耐受性,对死亡率、心血管终点和生活质量指标的影响没有差异。[3] 本研究旨在验证以下假设:Verapamil/维拉帕米的抗心律失常特性可能伴随着通过钙内流抑制来保护连接蛋白43(Cx43)蛋白。在一项体内研究中,Sprague-Dawley大鼠通过闭塞左前降支(LAD)冠状动脉45分钟诱导心肌缺血性心律失常。缺血前,将钙通道拮抗剂维拉帕米静脉注射到股静脉。还测定了维拉帕米对Bay K8644(一种钙通道激动剂)诱导的心律失常的影响。在一项离体研究中,离体心脏接受了最初10分钟的基线正常灌注,并在维拉帕米存在或不存在的情况下接受了高钙灌注。通过心电图(ECG)测量心律失常,通过免疫组织化学和蛋白质印迹测定Cx43蛋白。心肌缺血前服用维拉帕米可显著降低室性心律失常的发生率和心律失常总评分,同时降低心率、平均动脉压和左心室收缩压。维拉帕米还抑制了Bay K8644和高钙灌注引起的心律失常。异搏定对缺血性心律失常评分的影响被Cx43蛋白解偶联剂庚醇和Cx43通道抑制剂Gap 26消除。免疫组织化学数据显示,维拉帕米可以阻止缺血诱导的Cx43再分布和免疫染色减少。此外,在体内心肌缺血或离体高钙灌注后,通过蛋白质印迹法测定的Cx43蛋白表达减少,并在维拉帕米给药后得以保留。我们的数据表明,维拉帕米可能通过抑制钙内流、抑制耗氧量以及同时保护Cx43蛋白来发挥抗心律失常作用[5]。 |
| 酶活实验 |
方法:采用EverFluor FL维拉帕米(EFV)作为维拉帕米的荧光探针,通过大鼠颈总动脉输注研究其在BRB中的转运。分别用TR-iBRB2细胞和RPE-J细胞研究了EFV在内外BRB的转运。
结果:在细胞不可渗透化合物的弱信号期间,视网膜组织中检测到EFV信号。在TR-iBRB2细胞中,EFV的定位不同于溶酶体运动剂LysoTracker®Red的定位,并且不会因NH4Cl的急性处理而改变。在RPE-J细胞中,部分观察到EFV的点状分布,经NH4Cl急性处理后,这种分布有所减少。TR-iBRB2细胞对EFV的摄取是温度依赖性的,与膜电位和pH无关,NH4Cl处理显著降低了EFV的吸收,而不同的细胞外pH和V-ATP酶抑制剂没有显著影响。TR-iBRB2细胞对EFV的摄取被阳离子药物抑制,维拉帕米以浓度依赖的方式抑制,IC50为98.0μM。[4]
|
| 细胞实验 |
维拉帕米的抗心律失常作用是在人们意识到它是一种钙离子拮抗剂之前观察到的。静脉注射维拉帕米在终止阵发性往复式房室心动过速方面非常有效,无论是与预激有关还是仅涉及房室结。它持续减缓和规范房颤患者的心室反应,通常会增加房扑患者的房室结传导阻滞程度,尽管偶尔会导致窦性心律的恢复。口服可用于预防房室折返性心动过速,也可用于调节房颤患者的房室结反应。室性心动过速的有利反应是罕见的,然后在特定的良性品种中可见。维拉帕米是终止阵发性室上性心动过速的首选药物[2]。
TR-iBRB2细胞和RPE-J细胞的共聚焦显微镜[4] TR-iBRB2细胞和RPE-J细胞是永生化的大鼠视网膜毛细血管内皮细胞和视网膜色素上皮细胞,用作内外BRB模型细胞系,并在5℃下接种 × 103 and 7 × 103 分别在BioCoat™胶原蛋白I Cellware 8孔培养载玻片上培养细胞/孔,并在33°C、5%CO2/空气下培养。培养48小时后,通过加入细胞外液(ECF)启动摄取试验,该缓冲液含有EverFluor FL维拉帕米(EFV)(1μM)或LTR(TR-iBRB2细胞为300 nM,RPE-J细胞为600 nM),这些浓度是通过参考之前的报告来设定的。通过用冰冷的ECF缓冲液洗涤细胞三次,在指定时间终止测定。用4%多聚甲醛固定细胞后,用LSM700对细胞进行共聚焦显微镜观察,如别处所述。 细胞摄取研究[4] 参考之前的报告,使用TR-iBRB2细胞进行了体外摄取分析,细胞在33°C、5%CO2/空气的条件下在胶原蛋白涂层的24孔板上培养。通过在37°C下加入含有EverFluor FL维拉帕米(EFV)(200μL中1μM)的ECF缓冲液开始摄取试验,并用冰冷的ECF缓冲剂洗涤孔三次终止摄取试验。加入ECF缓冲液(200μL/孔)后,在超声波均质器中均质化细胞,用多模式微孔板读数器系统测量细胞蛋白含量和EFV的荧光强度。EFV摄取通过方程式1表示为细胞与培养基(C/M)比。EverFluor FL维拉帕米(EFV)在抑制剂存在下的摄取通过方程式2表示为荧光强度比(FI比)。非线性最小二乘回归分析程序MULTI用于测定EverFluor FL Verapamil (EFV)摄取中维拉帕米的50%抑制浓度(IC50),并将数据拟合到方程3中。在体外抑制分析中,参考我们之前关于TR-iBRB2细胞转运维拉帕米的报告,将抑制剂的浓度设置为500μM,P和Pmax是有和没有抑制剂的FI比率,Pmin是有抑制剂的抑制剂不敏感FI比率。[I]和n分别是抑制剂浓度和希尔系数。 |
| 动物实验 |
The present study was to test the hypothesis that anti-arrhythmic properties of Verapamil may be accompanied by preserving connexin43 (Cx43) protein via calcium influx inhibition. In an in vivo study, myocardial ischemic arrhythmia was induced by occlusion of the left anterior descending (LAD) coronary artery for 45 min in Sprague-Dawley rats. Verapamil, a calcium channel antagonist, was injected i.v. into a femoral vein prior to ischemia. Effects of verapamil on arrhythmias induced by Bay K8644 (a calcium channel agonist) were also determined. In an ex vivo study, the isolated heart underwent an initial 10 min of baseline normal perfusion and was subjected to high calcium perfusion in the absence or presence of Verapamil . Cardiac arrhythmia was measured by electrocardiogram (ECG) and Cx43 protein was determined by immunohistochemistry and western blotting. Administration of verapamil prior to myocardial ischemia significantly reduced the incidence of ventricular arrhythmias and total arrhythmia scores, with the reductions in heat rate, mean arterial pressure and left ventricular systolic pressure. Verapamil also inhibited arrhythmias induced by Bay K8644 and high calcium perfusion. Effect of Verapamil on ischemic arrhythmia scores was abolished by heptanol, a Cx43 protein uncoupler and Gap 26, a Cx43 channels inhibitor. Immunohistochemistry data showed that ischemia-induced redistribution and reduced immunostaining of Cx43 were prevented by verapamil. In addition, diminished expression of Cx43 protein determined by western blotting was observed following myocardial ischemia in vivo or following high calcium perfusion ex vivo and was preserved after Verapamil administration. Our data suggest that verapamil may confer an anti-arrhythmic effect via calcium influx inhibition, inhibition of oxygen consumption and accompanied by preservation of Cx43 protein[5].
Common Carotid Artery Infusion Analysis [4] In vivo distribution analysis of EverFluor FL Verapamil (EFV) to the retina was conducted by modifying the in situ brain perfusion method reported previously. In the anesthetized Wistar rats with pentobarbital (50 mg/kg), the right external carotid artery was ligated by a silk thread. After ligating the right common carotid artery, polyethylene tube was inserted into the right common carotid artery just below the bifurcation of the external carotid artery, and fixed by silk thread. The dosage conditions for fluorescent compounds were verified by referring previous reports that examined conditions without toxicity. Ringer-HEPES solution containing EverFluor FL Verapamil (EFV) (400 μg/3.5 mL), Rho-D (4 mg/3.5 mL) or LTR (400 μg/3.5 mL) warmed at 37°C was infused into the pterygopalatine artery and the internal carotid artery at a constant flow rate (0.85 mL/min) with an infusion pump, and this flow rate was set to avoid damaging the barrier structure with consideration for the blood-flow rate of the retina (0.7 mL/(min·g retina)). At the end of the infusion, the rats were decapitated, and their right eyeballs were immediately collected to be soaked in phosphate-buffered saline (PBS) containing 4% paraformaldehyde for 3 h, followed by soaking in PBS containing 30% sucrose at 4°C. The tissues were then fixed in the optimal cutting temperature compound, and tissue slices were prepared by means of a cryostat. Tissue slices mounted on glass slides were treated with 4′,6-diamidino-2-phenylindole (DAPI) and VECTASHIELD mounting medium, to be examined with a confocal microscope as described elsewhere. The excitation wavelength of 488 nm was used for EverFluor FL Verapamil (EFV), and 543 nm was used for LTR and Rho-D. In vivo Arrhythmia Study [5] Verapamil (1 mg/kg) was injected i.v. into a femoral vein 10 min prior to ischemia. A sham group underwent the same surgical procedures, except the suture underneath the LAD was left untied. In another series of experiment, arrhythmia was induced by Bay K8644, an L-type calcium channel agonist, at a dose of 0.1 mg/kg given i.v. into the FV. Verapamil (1 mg/kg) was administered 10 min prior to Bay K8644. All injections were performed within 30 sec. Heart Isolation and Perfusion [5] Each heart underwent an initial 10 min of baseline normal perfusion and was subjected to perfusion at 37°C for 45 min. The hearts were then randomly divided into three groups: Control group (normal calcium perfusion) (1.5 mmol/L), high calcium group (high calcium perfusion) (3.3 mmol/L) and Verapamil group (high calcium plus verapamil perfusion) (3.3 mmol/L calcium +3 µmol/L Verapamil ). For measurement of arrhythmias, the ECG was continuously monitored during the entire perfusion period and the incidence of arrhythmias was evaluated. Measurement of ECG and Determination of Arrhythmia Score [5] Anti-arrhythmic properties of Verapamil were determined in an animal model of ischemia-induced arrhythmia or in the presence of Bay K8644 or heptanol or Gap 26, respectively. The occurrence of cardiac arrhythmias throughout the 45 min was compared by ECG recording. For analysis of arrhythmia in Langendorff-perfused rat heart, each rat heart was continuously monitored with a positive electrode attached to the heart and a negative electrode to the aorta. After 10 min of a baseline normal perfusion period, incidences of arrhythmias under different concentrations of Ca2+ in the initial 45 min of perfusion period were compared. To enable a good quantitative comparison, 45 min of an ischemia period were divided into 15 3-min intervals in an in vivo arrhythmia evaluation and 45 min of a perfusion period was divided into 15 3-min intervals in an ex vivo arrhythmia investigation. Arrhythmia scores were evaluated as described previously. PVC ≤10/3-min period was recorded as 0; 10–50 PVC/3-min period was recorded as 1; ≥50 PVC/3-min period was recorded as 2; 1 episode of VF/3-min period was recorded as 3; 2–5 episodes of VF/3-min period was recorded as 4; and ≥5 episodes of VF/3-min period was recorded as 5. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
More than 90% of orally administered verapamil is absorbed - despite this, bioavailability ranges only from 20% to 30% due to rapid biotransformation following first-pass metabolism in the portal circulation. Absorption kinetic parameters are largely dependent on the specific formulation of verapamil involved. Immediate-release verapamil reaches peak plasma concentrations (i.e. Tmax) between 1-2 hours following administration, whereas sustained-release formulations tend to have a Tmax between 6 - 11 hours. AUC and Cmax values are similarly dependent upon formulation. Chronic administration of immediate-release verapamil every 6 hours resulted in plasma concentrations between 125 and 400 ng/mL. Steady-state AUC0-24h and Cmax values for a sustained-release formulation were 1037 ng∙h/ml and 77.8 ng/mL for the R-isomer and 195 ng∙h/ml and 16.8 ng/mL for the S-isomer, respectively. Interestingly, the absorption kinetics of verapamil are highly stereospecific - following oral administration of immediate-release verapamil every 8 hours, the relative systemic availability of the S-enantiomer compared to the R-enantiomer was 13% after a single dose and 18% at steady-state. Approximately 70% of an administered dose is excreted as metabolites in the urine and ≥16% in the feces within 5 days. Approximately 3% - 4% is excreted in the urine as unchanged drug. Verapamil has a steady-state volume of distribution of approximately 300L for its R-enantiomer and 500L for its S-enantiomer. Systemic clearance following 3 weeks of continuous treatment was approximately 340 mL/min for R-verapamil and 664 mL/min for S-verapamil. Of note, apparent oral clearance appears to vary significantly between single dose and multiple-dose conditions. The apparent oral clearance following single doses of verapamil was approximately 1007 mL/min for R-verapamil and 5481 mL/min for S-verapamil, whereas 3 weeks of continuous treatment resulted in apparent oral clearance values of approximately 651 mL/min for R-verapamil and 2855 mL/min for S-verapamil. /MILK/ Breast milk: Verapamil may appear in breast milk. /MILK/ Verapamil is excreted into breast milk. A daily dose of 240 mg produced milk levels that were approx 23% of maternal serum. Serum levels in the infant were 2.1 ng/mL but could not be detected (<1 ng/mL) 38 hr after treatment was stopped. ... In a second case, a mother was treated with 80 mg 3 times/day for hypertension for 4 wk prior to the determination of serum & milk concns. Steady-state concentrations of verapamil and the metabolite, norverapamil, in milk were 25.8 and 8.8 ng/mL, respectively. These values were 60% and 16% of the concns in plasma. The investigators estimated that the breast-fed child received <0.01% of the mother's dose. Neither verapamil nor the metabolite could be detected in the plasma of the child. The pharmacokinetics and hemodynamic effects of a combination of verapamil and trandolapril were studied in 20 patients with hypertension (ages 29-71 yr), 10 of whom also had fatty liver disease, who received a sustained-release oral capsule containing 180 mg verapamil and 1 mg trandolapril once daily for 7 days. For verapamil, no statistically significant differences were seen between patients with and without fatty liver with regard to Cmax (110.5 vs 76.5 ug/L), plasma AUC from 0-24 hr (1260.6 vs 941.2 ug/L hr), and elimination half-life (9.8 vs 9.2 hr). An open, randomized, single dose study of the effects of food on the bioavailability of sustained-release (SR) verapamil hydrochloride (Isoptin) was conducted in 12 healthy volunteers (aged 19-65 yr) who received 240 mg of the SR preparation while fasting or with food and a conventional preparation while fasting. Although the elimination half-life of SR verapamil was unchanged, the time to maximum concentration was prolonged and the area under the concentration-time curve (AUC) was 80% of the regular preparation. Concomitant food administration prolonged the time to maximum concentration from 7.3+-3.4 to 11.7+-6.3 h but had little effect on the maximum concentration, half-life or AUC of SR verapamil. For more Absorption, Distribution and Excretion (Complete) data for Verapamil (21 total), please visit the HSDB record page. Metabolism / Metabolites Verapamil is extensively metabolized by the liver, with up to 80% of an administered dose subject to elimination via pre-systemic metabolism - interestingly, this first-pass metabolism appears to clear the S-enantiomer of verapamil much faster than the R-enantiomer. The remaining parent drug undergoes O-demethylation, N-dealkylation, and N-demethylation to a number of different metabolites via the cytochrome P450 enzyme system. Norverapamil, one of the major circulating metabolites, is the result of verapamil's N-demethylation via CYP2C8, CYP3A4, and CYP3A5, and carries approximately 20% of the cardiovascular activity of its parent drug. The other major pathway involved in verapamil metabolism is N-dealkylation via CYP2C8, CYP3A4, and CYP1A2 to the D-617 metabolite. Both norverapamil and D-617 are further metabolized by other CYP isoenzymes to various secondary metabolites. CYP2D6 and CYP2E1 have also been implicated in the metabolic pathway of verapamil, albeit to a minor extent. Minor pathways of verapamil metabolism involve its O-demethylation to D-703 via CYP2C8, CYP2C9, and CYP2C18, and to D-702 via CYP2C9 and CYP2C18. Several steps in verapamil's metabolic pathway show stereoselective preference for the S-enantiomer of the given substrate, including the generation of the D-620 metabolite by CYP3A4/5 and the D-617 metabolite by CYP2C8. Metabolites: The main metabolite is norverapamil which has an elimination half-life very similar to that of the parent compound, ranging from 4 to 8 hours. Verapamil undergoes an extensive hepatic metabolism. Due to a large hepatic first-pass effect, bioavailability does not exceed 20 - 35% in normal subjects. Twelve metabolites have been described. The main metabolite is norverapamil and the others are various N- and 0-dealkylated metabolites. Elimination by route of exposure: Kidney: About 70% of the administered dose is excreted in urine within 5 days as metabolites, of which 3-4% is excreted as unchanged drug. Feces: About 16% of the ingested dose is excreted within 5 days in feces as metabolites. Breast milk: Verapamil may appear in breast milk. Verapamil yields in the dog: 5-(3,4-dimethoxyphenethylamino)-2 -(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile; 2-(3,4-dimethoxyphenyl)-5 -(n-(4-hydroxy-3-methoxyphenethyl)methylamino)-2-isopropylvaleronitrile, and 2-(3,4-dimethoxyphenyl)-2-isopropyl-5-methylaminovaleronitrile. The latter was also found in rats. /From table/ /salt not specified/ Verapamil and its major metabolite norverapamil were identified to be both mechanism-based inhibitors and substrates of CYP3A and reported to have non-linear pharmacokinetics in clinic. Metabolic clearances of verapamil and norverapmil as well as their effects on CYP3A activity were firstly measured in pooled human liver microsomes. The results showed that S-isomers were more preferential to be metabolized than R-isomers for both verapamil and norverapamil, and their inhibitory effects on CYP3A activity were also stereoselective with S-isomers more potent than R-isomers. A semi-physiologically based pharmacokinetic model (semi-PBPK) characterizing mechanism-based auto-inhibition was developed to predict the stereoselective pharmacokinetic profiles of verapamil and norverapamil following single or multiple oral doses. Good simulation was obtained, which indicated that the developed semi-PBPK model can simultaneously predict pharmacokinetic profiles of S-verapamil, R-verapamil, S-norverapamil and R-norverapamil. Contributions of auto-inhibition to verapamil and norverapamil accumulation were also investigated following the 38th oral dose of verapamil sustained-release tablet (240 mg once daily). The predicted accumulation ratio was about 1.3-1.5 fold, which was close to the observed data of 1.4-2.1-fold. Finally, the developed semi-PBPK model was further applied to predict drug-drug interactions (DDI) between verapamil and other three CYP3A substrates including midazolam, simvastatin, and cyclosporine A. Successful prediction was also obtained, which indicated that the developed semi-PBPK model incorporating auto-inhibition also showed great advantage on DDI prediction with CYP3A substrates. The biotransformation pathway of verapamil, a widely prescribed calcium channel blocker, was investigated by electrochemistry (EC) coupled online to liquid chromatography (LC) and electrospray mass spectrometry (ESI-MS). Mimicry of the oxidative phase I metabolism was achieved in a simple amperometric thin-layer cell equipped with a boron-doped diamond (BDD) working electrode. Structures of the electrochemically generated metabolites were elucidated on the basis of accurate mass data and additional MS/MS experiments. We were able to demonstrate that all of the most important metabolic products of the calcium antagonist including norverapamil (formed by N-demethylation) can easily be simulated using this purely instrumental technique. Furthermore, newly reported metabolic reaction products like carbinolamines or imine methides become accessible. The results obtained by EC were compared with conventional in vitro studies by conducting incubations with rat as well as human liver microsomes (RLMs, HLMs). Both methods showed good agreement with the data from EC/LC/MS. Thus, it can be noted that EC is very well-suited for the simulation of the oxidative metabolism of verapamil. In summary, this study confirms that EC/LC/MS can be a powerful tool in drug discovery and development when applied complementary to established in vitro or in vivo approaches. Mechanism-based inactivation (MBI) of cytochrome P450 (CYP) 3A by verapamil and the resulting drug-drug interactions have been studied in vitro, but the inhibition of verapamil on its own metabolic clearance in clinic, namely auto-inhibition of verapamil metabolism, has never been reproduced in vitro. This paper aimed to evaluate the utility of gel entrapped rat hepatocytes in reflecting such metabolic auto-inhibition using hepatocyte monolayer as a control. Despite being a similar concentration- and time-dependent profile, auto-inhibition of verapamil metabolism showed apparent distinctions between the two culture models. Firstly, gel entrapped hepatocytes were more sensitive to such inhibition, which could be largely due to their higher CYP3A activity detected by the formation rates of 6-beta-hydroxy testosterone and 1'-hydroxy midazolam. Furthermore, the inhibitory effect of ketoconazole and verapamil on CYP 3A activity as well as the reduction of verapamil intrinsic clearance (CL(int)) by ketoconazole was only observed in gel-entrapped hepatocytes. In this respect, the involvement of CYP3A in auto-inhibition of verapamil metabolism could be illustrated in gel-entrapped hepatocytes but not in hepatocyte monolayer. All of these results indicated that hepatocytes of gel entrapment reflected more of verapamil metabolic auto-inhibition than hepatocyte monolayer and could serve as a suitable system for investigating drug metabolism. Verapamil has known human metabolites that include 2-(3,4-dimethoxyphenyl)acetaldehyde, Norverapamil, D-702, M9 (D-703), and D-617. Route of Elimination: Approximately 70% of an administered dose is excreted as metabolites in the urine and 16% or more in the feces within 5 days. About 3% to 4% is excreted in the urine as unchanged drug. Half Life: 2.8-7.4 hours Biological Half-Life Single-dose studies of immediate-release verapamil have demonstrated an elimination half-life of 2.8 to 7.4 hours, which increases to 4.5 to 12.0 hours following repetitive dosing. The elimination half-life is also prolonged in patients with hepatic insufficiency (14 to 16 hours) and in the elderly (approximately 20 hours). Intravenously administered verapamil has rapid distribution phase half-life of approximately 4 minutes, followed by a terminal elimination phase half-life of 2 to 5 hours. The pharmacokinetics of verapamil and its metabolite, norverapamil, were studied in 10 patients (ages 19-69 yr) with portal hypertension and in 6 healthy subjects (ages 21-69 yr) who received an oral dose of 80 mg verapamil hydrochloride (Isoptin). The terminal phase half-life of verapamil was 210 hr in controls and 1384 hr in patients. A toxicokinetic study performed in two cases showed plasma half lives of 7.9 and 13.2 hours, total body clearances of 425 and 298 mL/min. ... In Vivo Distribution Analysis of EverFluor FL Verapamil (EFV) to the Retina [4] Common carotid artery infusion was conducted to investigate the distribution of EFV to the retina. In confocal microscopy, the signal of EFV (green) was uniformly detected in the region from inner limiting membrane (ILM) to the outer plexiform layer (OPL) (Fig. 1a), while a weak fluorescence signal was detected for Rho-D which is a cell impermeable substrate (data not shown). In addition, a strong fluorescence signal of EFV was detected in the photoreceptor outer segment (POS) (Fig. 1a), and the punctate signal of EFV was partially detected in the RPE (Fig. 1b, arrow head). In Vitro Distribution Analysis of EverFluor FL Verapamil (EFV) [4] Confocal microscopy was performed to investigate the subcellular localization of EFV in the model cell lines of the inner and outer BRB. In TR-iBRB2 cells, an in vitro model cell line of the inner BRB, the fluorescence signal of EFV was detected all over the cells, while the fluorescence signal of LTR had a punctate distribution pattern, showing that subcellular distribution pattern of EFV is different from that of LTR (Fig. 2a). In the case of acute treatment with NH4Cl, no prominent change was seen in the subcellular localization of EFV, while the punctate distribution pattern of LTR was reduced (Fig. 2b). In addition, EFV uptake by TR-iBRB2 cells was not significantly changed in the presence of bafilomycin A1, an inhibitor of vacuolar-type H+-ATPase (V-ATPase) (Fig. 2c). [4] In RPE-J cells, a punctate distribution pattern of LTR was observed, and this was reduced by acute treatment with NH4Cl (Fig. 3). The signal of EFV was observed all over the cells with a partial punctate distribution pattern, that merged with the punctate distribution pattern of LTR (Fig. 3a, arrow head), and this partial distribution was reduced by acute treatment with NH4Cl (Fig. 3). |
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Verapamil is the drug of choice for prevention and treatment of paroxysmal supraventricular tachycardia. Verapamil has been shown to be effective in the treatment of angina pectoris. Verapamil may be used as an alternative treatment for mild or moderate hypertension. HUMAN STUDIES: Verapamil has a vasodilating action on the vascular system. Toxic effects occur usually after a delay of 1 to 5 hours following ingestion. After IV injection, symptoms appear after a few minutes. The main cardiovascular symptoms are: bradycardia and atrioventricular block (in 82% of cases) hypotension and cardiogenic shock (in 78% of cases) cardiac arrest (in 18% of cases). Pulmonary edema may occur. Impairment of consciousness and seizures may occur and are related to a low cardiac output. Nausea and vomiting may be observed. Metabolic acidosis due to shock and hyperglycemia may occur. Verapamil is a calcium channel blocker and inhibits the entry of calcium through calcium channels into cardiovascular cells. Verapamil reduces the magnitude of the calcium current entry and decreases the rate of recovery of the channel. Verapamil decreases peripheral vascular and coronary resistance but it is a less potent vasodilator than nifedipine. In contrast, its cardiac effects are more prominent than those of nifedipine. At doses necessary to produce arterial vasodilatation, verapamil has much greater negative chronotropic, dromotropic and inotropic effects than nifedipine. At toxic doses, calcium channel inhibition by verapamil results in three principal effects: hypotension due to arterial vasodilatation, cardiogenic shock secondary to a negative inotropic effect, bradycardia and atrio-ventricular block. The therapeutic effects of verapamil on hypertension and angina pectoris are due to arterial systemic and coronary vasodilatation. The antiarrhythmic activity of verapamil is due to a delay in impulse transmission through the AV node by a direct action. Toxicity may occur after ingestion of 1 g. Verapamil was tested on human peripheral lymphocytes in vitro using micronucleus (MN) test. The MN frequencies showed increase after all treatment. The results of FISH analysis suggest that verapamil, separately or combined with ritodrine, shows to a larger extent aneugenic than clastogenic effect. ANIMAL STUDIES: Verapamil promotes atrial fibrillation in normal dogs. In swine, verapamil toxicity, as defined by a mean arterial pressure of 45% of baseline, was produced following an average verapamil infusion dose of 0.6 +/- 0.12 mg/kg. This dose produced an average plasma verapamil concentration of 728.1 +/- 155.4 ug/L. Hypertonic sodium bicarbonate reversed the hypotension and cardiac output depression of severe verapamil toxicity in a swine model. ECOTOXICITY STUDIES: Effects of long-term exposure of verapamil on mutagenic, hematological parameters and activities of the oxidative enzymes of Nile tilapia, Oreochromis niloticus were investigated for 60 days exposure at the concentrations of 0.29, 0.58 and 1.15 mg/L in the fish liver. The exposure resulted in significantly high micronuclei induction of peripheral blood cells. The indices of oxidative stress biomarkers (lipid peroxidation and carbonyl protein) showed elevated level. There was increase in the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione-S-transferase (GST). In other experiments, exposure to sub-lethal concentrations of verapamil (0.14, 0.29 and 0.57 mg/L) for period of 15, 30, 45 and 60 days, led to inhibition of acetylcholinesterase activities in the brain and muscle of the fish. Transcription of catalase (CAT), superoxide dismutase (SOD) and heat shock proteins 70 (hsp70) were up-regulated in both the tissues after the study period. In Carassius auratus, the behavioral alterations were observed in the form of respiratory difficulty and loss of body balance confirming the cardiovascular toxicity caused by verapamil at higher doses. In addition to affecting the cardiovascular system, verapamil also showed effects on the nervous system in the form of altered expression of parvalbumin. Acute exposure to verapamil significantly reduced the heart rate in the embryos and larvae of common carp (Cyprinus carpio). In the D. magna chronic toxicity test, several parameters, such as the survival percentage, the body length of D. magna, the time of first reproduction, and the number of offspring per female, were adversely affected during the exposure to 4.2 mg/L verapamil. During the 24-hr short-term exposure, verapamil caused a downregulated expression of the CYP4 and CYP314 genes. During the 21-day long-term exposure, verapamil significantly reduced the expression level of the Vtg gene, a biomarker of the reproduction ability in an oviparous animal. Verapamil inhibits voltage-dependent calcium channels. Specifically, its effect on L-type calcium channels in the heart causes a reduction in ionotropy and chronotropy, thuis reducing heart rate and blood pressure. Verapamil's mechanism of effect in cluster headache is thought to be linked to its calcium-channel blocker effect, but which channel subtypes are involved is presently not known. Toxicity Data LD50: 8 mg/kg (Intravenous, Mouse) (A308) Interactions Drug interactions: protein-bound drugs Drug Interactions: beta-adrenergic blocking agents Drug Interactions: digoxin Drug Interactions: hypotensive agents For more Interactions (Complete) data for Verapamil (42 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Mouse ip 68 mg/kg /Verapamil hydrochloride/ LD50 Rat ip 67 mg/kg /Verapamil hydrochloride/ LD50 Rat oral 114 mg/kg /Verapamil hydrochloride/ LD50 Mouse iv 7.6 mg/kg /Verapamil hydrochloride/ For more Non-Human Toxicity Values (Complete) data for Verapamil (14 total), please visit the HSDB record page. |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Anti-Arrhythmia Agents; Calcium Channel Blockers; Vasodilator Agents /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Verapamil hydrochloride is included in the database. Oral calcium-channel blocking agents are considered the drugs of choice for the management of Prinzmetal variant angina. A nondihydropyridine calcium-channel blocker (e.g., diltiazem, verapamil) also has been recommended in patients with unstable angina who have continuing or ongoing ischemia when therapy with beta-blocking agents and nitrates is inadequate, not tolerated, or contraindicated and when severe left ventricular dysfunction, pulmonary edema, or other contraindications are not present. In the management of unstable or chronic stable angina pectoris, verapamil appears to be as effective as beta-adrenergic blocking agents (e.g., propranolol) and/or oral nitrates. In unstable or chronic stable angina pectoris, verapamil may reduce the frequency of attacks, allow a decrease in sublingual nitroglycerin dosage, and increase the patient's exercise tolerance. /Included in US product label/ Verapamil is used for rapid conversion to sinus rhythm of paroxysmal supraventricular tachycardia (PSVT), including tachycardia associated with Wolff-Parkinson-White or Lown-Ganong-Levine syndrome; the drug also is used for control of rapid ventricular rate in nonpreexcited atrial flutter or fibrillation. The American College of Cardiology/American Heart Association/Heart Rhythm Society (ACC/AHA/HRS) guideline for the management of adult patients with supraventricular tachycardia recommends the use of verapamil in the treatment of various SVTs (e.g., atrial flutter, junctional tachycardia, focal atrial tachycardia, atrioventricular nodal reentrant tachycardia (AVNRT)); in general, IV verapamil is recommended for acute treatment, while oral verapamil is recommended for ongoing management of these arrhythmias. /Included in the US product label/ For more Therapeutic Uses (Complete) data for Verapamil (14 total), please visit the HSDB record page. Drug Warnings ...Concurrent treatment /of verapamil & beta-blockers/ in those with impaired left ventricular function could be dangerous if...a 10-15% depression in myocardial function takes place. /Salt not specified/ ...Absolute contraindications to the use of verapamil (the acute stage of myocardial infarction, complete atrioventricular block, cardiogenic shock, overt heart failure)...should not be injected together with a beta-adrenergic blocking agent, or within 3 times the half-life of that agent. /Salt not specified/ The basic physiologic actions of verapamil may lead to serious adverse effects. /Salt not specified/ Maternal Medication usually Compatible with Breast-Feeding: Verapamil: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/ /Salt not specified/ For more Drug Warnings (Complete) data for Verapamil (23 total), please visit the HSDB record page. Pharmacodynamics Verapamil is an L-type calcium channel blocker with antiarrhythmic, antianginal, and antihypertensive activity. Immediate-release verapamil has a relatively short duration of action, requiring dosing 3 to 4 times daily, but extended-release formulations are available that allow for once-daily dosing. As verapamil is a negative inotropic medication (i.e. it decreases the strength of myocardial contraction), it should not be used in patients with severe left ventricular dysfunction or hypertrophic cardiomyopathy as the decrease in contractility caused by verapamil may increase the risk of exacerbating these pre-existing conditions. 2-(3,4-dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-(propan-2-yl)pentanenitrile is a tertiary amino compound that is 3,4-dimethoxyphenylethylamine in which the hydrogens attached to the nitrogen are replaced by a methyl group and a 4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl group. It is a tertiary amino compound, an aromatic ether, a polyether and a nitrile. Verapamil is a phenylalkylamine calcium channel blocker used in the treatment of high blood pressure, heart arrhythmias, and angina, and was the first calcium channel antagonist to be introduced into therapy in the early 1960s. It is a member of the non-dihydropyridine class of calcium channel blockers, which includes drugs like [diltiazem] and [flunarizine], but is chemically unrelated to other cardioactive medications. Verapamil is administered as a racemic mixture containing equal amounts of the S- and R-enantiomer, each of which is pharmacologically distinct - the S-enantiomer carries approximately 20-fold greater potency than the R-enantiomer, but is metabolized at a higher rate. Verapamil is a Calcium Channel Blocker. The mechanism of action of verapamil is as a Calcium Channel Antagonist, and Cytochrome P450 3A4 Inhibitor, and Cytochrome P450 3A Inhibitor, and P-Glycoprotein Inhibitor. Verapamil is a first generation calcium channel blocker used for treatment of hypertension, angina pectoris and superventricular tachyarrhythmias. Verapamil has been linked to a low rate of serum enzyme elevations during therapy and to rare instances of clinically apparent acute liver injury. Verapamil has been reported in Teichospora striata and Schisandra chinensis with data available. LOTUS - the natural products occurrence database Verapamil is a phenylalkylamine calcium channel blocking agent. Verapamil inhibits the transmembrane influx of extracellular calcium ions into myocardial and vascular smooth muscle cells, causing dilatation of the main coronary and systemic arteries and decreasing myocardial contractility. This agent also inhibits the drug efflux pump P-glycoprotein which is overexpressed in some multi-drug resistant tumors and may improve the efficacy of some antineoplastic agents. VERAPAMIL is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 1981 and has 4 approved and 16 investigational indications. Verapamil is only found in individuals that have used or taken this drug. Verapamil is a calcium channel blocker that is a class IV anti-arrhythmia agent. [PubChem]Verapamil inhibits voltage-dependent calcium channels. Specifically, its effect on L-type calcium channels in the heart causes a reduction in ionotropy and chronotropy, thuis reducing heart rate and blood pressure. Verapamil's mechanism of effect in cluster headache is thought to be linked to its calcium-channel blocker effect, but which channel subtypes are involved is presently not known. [PubChem] Calcium channel antagonists can be quite toxic. In the management of poisoning, early recognition is critical. Calcium channel antagonists are frequently prescribed, and the potential for serious morbidity and mortality with over dosage is significant. Ingestion of these agents should be suspected in any patient who presents in an overdose situation with unexplained hypotension and conduction abnormalities. The potential for toxicity should be noted in patients with underlying hepatic or renal dysfunction who are receiving therapeutic doses. (A7844). A calcium channel blocker that is a class IV anti-arrhythmia agent. |
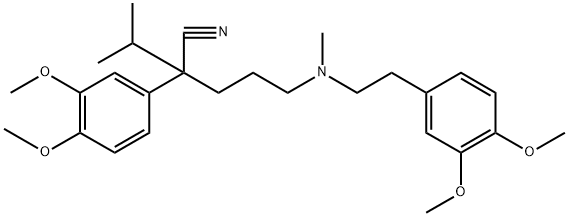
| 分子式 |
C27H38N2O4
|
|---|---|
| 分子量 |
454.61
|
| 精确质量 |
454.283
|
| 元素分析 |
C, 71.34; H, 8.43; N, 6.16; O, 14.08
|
| CAS号 |
52-53-9
|
| 相关CAS号 |
Verapamil hydrochloride;152-11-4; 38321-02-7 (dexverapamil)
|
| PubChem CID |
2520
|
| 外观&性状 |
Viscous, pale yellow oil
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
586.2±50.0 °C at 760 mmHg
|
| 熔点 |
25°C
|
| 闪点 |
308.3±30.1 °C
|
| 蒸汽压 |
0.0±1.6 mmHg at 25°C
|
| 折射率 |
1.526
|
| LogP |
3.9
|
| tPSA |
63.95
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
13
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
606
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(C)C(CCCN(C)CCC1=CC(=C(C=C1)OC)OC)(C#N)C2=CC(=C(C=C2)OC)OC
|
| InChi Key |
SGTNSNPWRIOYBX-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C27H38N2O4/c1-20(2)27(19-28,22-10-12-24(31-5)26(18-22)33-7)14-8-15-29(3)16-13-21-9-11-23(30-4)25(17-21)32-6/h9-12,17-18,20H,8,13-16H2,1-7H3
|
| 化学名 |
2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile
|
| 别名 |
NSC-135784; NSC 135784; VERAPAMIL; 52-53-9; Iproveratril; Dilacoran; Vasolan; Isoptimo; Isoptin; Verapamilo; Verapamil; Verapamilum; D-365;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~219.97 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.50 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1997 mL | 10.9984 mL | 21.9969 mL | |
| 5 mM | 0.4399 mL | 2.1997 mL | 4.3994 mL | |
| 10 mM | 0.2200 mL | 1.0998 mL | 2.1997 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。