| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
sPLA2; 5-HT1A Receptor
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Vilazodone 对人 5-HT1A 受体的 IC50 为 0.2 nM,对 SERT 的 IC50 为 0.5 nM。 Vilazodone 对人类重组体以及大鼠、豚鼠、小鼠和狨猴天然组织 5-HT1A 受体表现出高亲和力 (pKi ≥ 9.3)。细胞测定:施用 5-HT1A 受体激动剂会产生一种特征性行为综合征,包括姿势变化、后肢缩小、头部摆动、震颤、前爪踩踏和拉尾。在大鼠超声发声测试中,给药后 120 和 210 分钟,维拉佐酮(55 mg/kg po)抑制应激诱导的发声。维拉佐酮(20-40 mg/kg ip),急性或预防性给药(行为测试前 1 周),减弱的压力会诱发增强的惊吓,但对高架十字迷宫中的压力增强的焦虑反应没有影响。有趣的是,10 mg/kg 的较低剂量的维拉佐酮在惊吓反应中具有相反的作用,表明存在某种无法解释的双向效应,并且所有剂量都会产生惊吓引起的应激反应的增强,可能提示类似焦虑的反应。 1]。维拉佐酮也是治疗重度抑郁症的另一种选择。
|
||
| 体内研究 (In Vivo) |
Vilazodone 选择性增强大鼠前额皮质的血清素输出。通过对大鼠焦虑超声发声模型的行为评估,维拉佐酮证明了抗焦虑功效。维拉佐酮也显示出疗效,但仅在强迫游泳测试(假定的抑郁模型)中使用单剂量。 Vilazodone (1-10 mg/kg po) 剂量依赖性地取代体内 [3H]DASB(N,N-二甲基-2-(2-氨基-4-氰基苯硫基)苯甲胺)与大鼠皮质和海马的结合,表明vilazodone占据体内5-HT转运蛋白。维拉佐酮(10 mg/kg,口服)被证明可导致自由活动大鼠的细胞外 5-HT 增加 2 倍,但额叶皮层去甲肾上腺素或多巴胺水平没有变化。维拉佐酮在剂量高于 5 mg/kg 时会影响大鼠的惊吓应激增强。维拉佐酮 10 mg/kg 会增加大鼠惊吓应激的升高。维拉佐酮(20 和 40 毫克/千克)可阻断大鼠惊吓的应激增强。所有剂量的维拉佐酮都会增加大鼠惊恐应激的升高。 Vilazodone 对豚鼠中缝背核中 100 nM 的 5-HT 流出没有影响,但在 1 mM 时显着降低 5-HT 流出。维拉佐酮显着增加豚鼠中缝背核中 5-HT 的再摄取半衰期。
|
||
| 酶活实验 |
维拉唑酮的受体结合谱由Heinrich等人报道。维拉唑酮对人5‐HT1A受体的IC50为0.2 nM,对SERT的IC50为0.5 nM。在这些研究中,其最接近的交叉亲和性是多巴胺D3受体(IC50为71 nM),其次是5‐HT4受体(IC50为252 nM)。我们使用5‐HT1A受体激动剂[3H]8‐OH‐DPAT进行的内部放射性配体结合研究表明,维拉唑酮对人重组体和大鼠、豚鼠、小鼠和狨猴的天然组织5‐HT1A受体具有高亲和力(pKi≥9.3)(未发表的数据见表1)。相比之下,维拉唑酮取代了拮抗剂放射性配体[3H]WAY100635。(在Gpp(NH)p存在的情况下)与pKi值比使用[3H]8‐OH‐DPAT获得的值低2个log单位(表2)。这些数据表明,维拉唑酮优先结合人类5‐HT1A受体的高激动剂亲和力状态,表明该分子具有部分激动剂活性。据报道,用[3H]8‐OH‐DPAT和[3H]WAY100635测定的化合物对5‐HT1A受体的亲和力差异与其内在激动剂活性成正比。因此,考虑到对[3H]8‐OH‐DPAT cf. [3H]WAY100635测定的亲和力差异与内源性激动剂5‐HT观察到的相似,这些数据表明维拉唑酮将作为5‐HT1A受体的高效部分激动剂。这一假设在表达h5‐HT1A受体的Sf9细胞中得到了[35S] gtp - γ - s结合研究的支持,其中单一浓度的维拉唑酮(100nM)使基础结合增加了约70%的5‐HT1A受体激动剂8‐OH‐PIPAT产生的基础结合。然而,由于本研究仅使用单一浓度,因此无法准确测定h5‐HT1A受体的内在活性或功能效力。此后,对表达h5‐HT1A受体的HEK细胞进行了更广泛的研究(未发表的数据)。在这些研究中,维拉唑酮作为完全激动剂,与5‐HT相比,pEC50为9.0。[1]
|
||
| 细胞实验 |
施用 5-HT1A 受体激动剂会导致一种独特的行为综合征,包括头晃动、震颤、前爪踩踏、姿势异常、后肢缩小和拖尾。给药后 120 和 210 分钟,Vilazodone (55 mg/kg po) 抑制大鼠超声发声练习中应激诱导的发声。在行为测试前一周急性或预防性给药时,维拉佐酮(20-40 mg/kg ip)可抑制应激诱发的强化惊吓,但对高架十字迷宫中应激强化的焦虑反应没有影响。有趣的是,10 mg/kg 较低剂量的维拉佐酮以相反的方式影响惊吓反应,这表明尚未完全了解的双向效应。此外,所有剂量的药物都会增加惊吓引起的应激反应,这可能表明出现了类似焦虑的反应。维拉佐酮是重度抑郁症的另一种治疗选择。
|
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption
Vilazodone's bioavailability is 72% when taken with food. Route of Elimination 1% of the dose is recovered unchanged in the urine and 2% of the dose is recovered unchanged in the feces. Volume of Distribution Vilazodone's volume of distribution is unknown but large Clearance Clearance of vilazodone is 19.9-25.1L/h in patients with mild to moderate renal impairment compared to 26.4-26.9L/h in healthy controls. Vilazodone concentrations peak at a median of 4-5 hours (Tmax) after administration and decline with a terminal half-life of approximately 25 hours. The absolute bioavailability of vilazodone is 72% with food. Administration of VIIBRYD with food (high fat or light meal) increases oral bioavailability (Cmax increased by approximately 147-160%, and AUC increased by approximately 64-85%). View More
Metabolism / Metabolites Vilazodone is mainly metabolized by cytochrome P450(CYP)3A4 and also to a minor extent by CYP2C19 and CYP 2D6. Although the metabolic pathway for vilazodone has not been fully studied, a proposed mechanism for metabolism in rats was published in 2017. Viibryd is extensively metabolized through CYP and non-CYP pathways (possibly by carboxylesterase), with only 1% of the dose recovered in the urine and 2% of the dose recovered in the feces as unchanged vilazodone. CYP3A4 is primarily responsible for its metabolism among CYP pathways, with minor contributions from CYP2C19 and CYP2D6. In vitro studies with human microsomes and human hepatocytes indicate that vilazodone is unlikely to inhibit or induce the metabolism of other CYP (except for CYP2C8) substrates; and an in vivo study with probe substrates for CYP2C19, 2D6 and 3A4 showed vilazodone did not alter the pharmacokinetics of the probe substrates. However, an in vivo study with probe substrate for CYP2C19 demonstrated a minor induction of CYP2C19. Strong inhibitors of CYP3A4 (e.g., ketoconazole) can reduce the metabolism of vilazodone in vivo and increase exposure. Conversely, strong inducers of CYP3A4 (e.g., carbamazepine) can decrease vilazodone exposure. NIH; DailyMed. Current Medication Information for Viibryd (Vilazodone Hydrochloride) Tablet Viibryd (Vilazodone Hydrochloride) Kit (Revised: July 2014). Available from, as of July 30, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c55ccfb-c4cf-11df-851a-0800200c9a66 Biological Half-Life 25 hours. Other studies show a half life of 24±5.2h with a single 40mg dose and 28.9±3.2h with repeated doses. Vilazodone /has/ a terminal half-life of approximately 25 hours. Vilazodone’s pharmacokinetic activity (5–80 mg) is dose-proportional. The terminal half-life is approximately 25 hours. When vilazodone is taken with food, the drug’s absolute bioavailability is 72%. After daily dosing of vilazodone 40 mg under fed conditions, the mean maximum plasma concentration (Cmax) at steady state was 156 ng/mL, and the mean area-under-the-curve (AUC0–24 hr) concentration was 1,645 ng • hours/mL. When vilazodone was administered with a high-fat or light meal, the Cmax was increased by approximately 147% to 160%, and the AUC concentration was increased by approximately 64% to 85%. If vomiting occurs within 7 hours after administration, the drug’s absorption is decreased by about 25%; however, a replacement dose is not required. Vilazodone has a large volume of distribution (value unknown). It is approximately 96% to 99% protein-bound The drug is extensively metabolized in the liver, primarily via the cytochrome P450 (CYP) 3A4 isoenzyme. CYP2C19 and CYP2D6 are minor metabolic pathways. Non-CYP450 metabolism also occurs, possibly by carboxylesterase. Only 1% and 2% of the dose are recovered in urine and feces, respectively, as unchanged vilazodone. The presence of mild or moderate renal and hepatic impairment does not affect the clearance of vilazodone. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Vilazodone is a white to off-white solid that is formulated into film-coated tablets. Vilazodone is a combined selective serotonin-reuptake inhibitor and serotonin type 1-A (5-hydroxytryptamine (5-HT1A) receptor partial agonist. It is used for the treatment of major depressive disorder in adults. HUMAN EXPOSURE AND TOXICITY: In clinical trials toxic effects of vilazodone at 200-280 mg included serotonin syndrome, lethargy, restlessness, hallucinations, and disorientation. Serotonin syndrome, a potentially life-threatening toxicity has also been reported at therapeutic doses. Serotonin syndrome symptoms may include mental status changes (agitation, hallucinations, delirium, and coma), autonomic instability (tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (nausea, vomiting, diarrhea). While serotonin syndrome has been reported during vilazodone monotherapy, it is a particular concern when used with other serotonergic drugs and with drugs that impair metabolism of serotonin (in particular, monamine oxidase inhibitors (MAOIs). The concomitant use of vilazdone with MAOIs intended to treat psychiatric disorders is contraindicated. Vilazodone is also not approved for use in pediatric patients. Pooled analyses of short-term placebo-controlled studies of antidepressant drugs (selective serotonin reuptake inhibitors and others) showed that these drugs increase the risk of suicidal thinking and behavior in children, adolescents, and young adults with major depressive disorder and other psychiatric disorders. Also, some neonates exposed to serotonegic antidepressants (including vilazodone) late in the third trimester of pregnancy have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. In some cases, the clinical picture is consistent with serotonin syndrome. Infants exposed to vilazodone in pregnancy may also have an increased risk for persistent pulmonary hypertension of the newborn, a rare heart and lung condition associated with substantial neonatal morbidity and mortality. ANIMAL STUDIES: Vilazodone caused some developmental toxicity in rats, but was not teratogenic in rats or rabbits. When vilazodone was administered to pregnant rats at an oral dose of 30 times the maximum recommended human dose during the period of organogenesis and throughout pregnancy and lactation, the number of live born pups was decreased. There was an increase in early postnatal pup mortality, and among surviving pups there was decreased body weight, delayed maturation, and decreased fertility in adulthood. There was some maternal toxicity at this dose. Hepatotoxicity In premarketing studies, liver test abnormalities were uncommon in patients taking vilazodone (Likelihood score: E* (unproven but suspected rare cause of clinically apparent liver injury). View More
Effects During Pregnancy and Lactation Interactions Concomitant administration of vilazodone and moderate CYP3A4 inhibitors (e.g., erythromycin) can result in increased plasma vilazodone concentrations. During concurrent administration with moderate inhibitors of CYP3A4 (e.g., erythromycin), the dosage of vilazodone should be reduced to 20 mg once daily in patients experiencing intolerable adverse effects. Concomitant administration of vilazodone and potent CYP3A4 inhibitors (e.g., clarithromycin, ketoconazole) can increase plasma vilazodone concentrations by approximately 50%. The manufacturer states that the dosage of vilazodone should be reduced to 20 mg once daily if administered concomitantly with a potent CYP3A4 inhibitor. Potentially serious, sometimes fatal adverse reactions may occur in patients who are receiving or have recently received a monoamine oxidase (MAO) inhibitor and then initiate therapy with antidepressant(s) that are pharmacologically similar to vilazodone (e.g., SSRIs), or in those who received SSRI therapy shortly before initiation of an MAO inhibitor. Concomitant use of MAO inhibitors with vilazodone is contraindicated. In addition, at least 2 weeks should elapse between discontinuance of an MAO inhibitor and initiation of vilazodone and vice versa. Linezolid, an anti-infective agent that is also a reversible MAO inhibitor, has been associated with drug interactions resulting in serotonin syndrome, including some associated with SSRIs. Because of this potential risk, linezolid generally should not be used in patients receiving vilazodone. While the US Food and Drug Administration (FDA) has not received reports of serotonin syndrome with concomitant use of linezolid and vilazodone to date, the risk is considered comparable to that with SSRIs. However, the FDA states that certain life-threatening or urgent situations may necessitate immediate linezolid treatment in a patient receiving a serotonergic drug. In such emergency situations, the availability of alternative anti-infectives should be considered and the benefits of linezolid should be weighed against the risk of serotonin syndrome. If linezolid is indicated in such emergency situations, vilazodone must be immediately discontinued and the patient monitored for symptoms of CNS toxicity for 2 weeks or until 24 hours after the last linezolid dose, whichever comes first. Treatment with vilazodone may be resumed 24 hours after the last linezolid dose. If nonemergency use of linezolid is being planned for a patient receiving vilazodone, vilazodone should be withheld for at least 2 weeks prior to initiating linezolid. Treatment with vilazodone should not be initiated in a patient receiving linezolid; when necessary, vilazodone may be started 24 hours after the last linezolid dose. Antidote and Emergency Treatment /SRP:/ Immediate first aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on the left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention. /Poisons A and B/ Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3rd revised edition, Elsevier Mosby, St. Louis, MO 2007, p. 160 /SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if needed. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . Monitor for shock and treat if necessary ... . Anticipate seizures and treat if necessary ... . For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool ... . Cover skin burns with dry sterile dressings after decontamination ... . /Poisons A and B/ Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3rd revised edition, Elsevier Mosby, St. Louis, MO 2007, p. 160 /SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag valve mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias as necessary ... . Start IV administration of D5W TKO /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's (LR) if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Treat seizures with diazepam or lorazepam ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Poisons A and B/ Currance, P.L. Clements, B., Bronstein, A.C. (Eds).; Emergency Care For Hazardous Materials Exposure. 3rd revised edition, Elsevier Mosby, St. Louis, MO 2007, p. 160-1 No specific antidotes for vilazodone are known. In case of an overdose, provide supportive care, including close medical supervision and monitoring. Treatment should consist of those general measures employed in the management of overdosage with any drug. Consider the possibility of multiple drug overdose. Ensure an adequate airway, oxygenation, and ventilation. Monitor cardiac rhythm and vital signs. General supportive and symptomatic measures are also recommended. ... Removal of vilazodone by dialysis has not been studied; however, the high volume of distribution of vilazodone suggests that dialysis will not be effective in reducing vilazodone plasma concentrations. NIH; DailyMed. Current Medication Information for Viibryd (Vilazodone Hydrochloride) Tablet Viibryd (Vilazodone Hydrochloride) Kit (Revised: July 2014). Available from, as of July 30, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=4c55ccfb-c4cf-11df-851a-0800200c9a66 Populations at Special Risk Vilazodone has not been systematically evaluated in patients with seizure disorders; such patients were excluded from clinical studies. As with other antidepressants, vilazodone should be used with caution in patients with a history of seizure disorder American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2455 Protein Binding: 96-99%. |
||
| 参考文献 | |||
| 其他信息 |
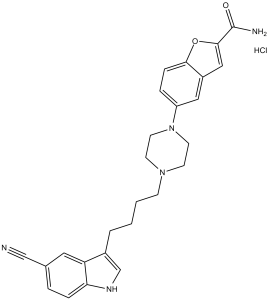
Vilazodone hydrochloride is a hydrochloride obtained by reaction of vilazodone with one equivalent of hydrochloric acid. Used for the treatment of major depressive disorder. It has a role as an antidepressant, a serotonin uptake inhibitor and a serotonergic agonist. It contains a vilazodone(1+).
A benzofuran, indole, and piperazine derivative that functions as a SEROTONIN UPTAKE INHIBITOR and partial SEROTONIN 5-HT1 RECEPTOR AGONIST. It is used as an ANTIDEPRESSIVE AGENT. See also: Vilazodone (has active moiety). |
| 分子式 |
C26H28CLN5O2
|
|
|---|---|---|
| 分子量 |
477.99
|
|
| 精确质量 |
477.193
|
|
| 元素分析 |
C, 65.33; H, 5.90; Cl, 7.42; N, 14.65; O, 6.69
|
|
| CAS号 |
163521-08-2
|
|
| 相关CAS号 |
Vilazodone-d8; 1794789-93-7; Vilazodone; 163521-12-8; Vilazodone carboxylic acid; 163521-19-5
|
|
| PubChem CID |
6918313
|
|
| 外观&性状 |
White to yellow solid powder
|
|
| 沸点 |
745.1ºC at 760 mmHg
|
|
| 熔点 |
279°C(lit.)
|
|
| 闪点 |
404.4ºC
|
|
| LogP |
5.718
|
|
| tPSA |
103.28
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
5
|
|
| 可旋转键数目(RBC) |
7
|
|
| 重原子数目 |
34
|
|
| 分子复杂度/Complexity |
729
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
Cl[H].O1C(C(N([H])[H])=O)=C([H])C2=C1C([H])=C([H])C(=C2[H])N1C([H])([H])C([H])([H])N(C([H])([H])C([H])([H])C([H])([H])C([H])([H])C2=C([H])N([H])C3C([H])=C([H])C(C#N)=C([H])C2=3)C([H])([H])C1([H])[H]
|
|
| InChi Key |
RPZBRGFNBNQSOP-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C26H27N5O2.ClH/c27-16-18-4-6-23-22(13-18)19(17-29-23)3-1-2-8-30-9-11-31(12-10-30)21-5-7-24-20(14-21)15-25(33-24)26(28)32;/h4-7,13-15,17,29H,1-3,8-12H2,(H2,28,32);1H
|
|
| 化学名 |
5-[4-[4-(5-cyano-1H-indol-3-yl)butyl]piperazin-1-yl]-1-benzofuran-2-carboxamide;hydrochloride
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (5.23 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (5.23 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (5.23 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.0921 mL | 10.4605 mL | 20.9209 mL | |
| 5 mM | 0.4184 mL | 2.0921 mL | 4.1842 mL | |
| 10 mM | 0.2092 mL | 1.0460 mL | 2.0921 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05948579 | Not yet recruiting | Drug: Intervention B Vilazodone Hydrochloride (HCl) Drug: Intervention B Placebo |
Post Traumatic Stress Disorder | U.S. Army Medical Research and Development Command |
August 2023 | Phase 2 |
| NCT05422612 | Recruiting | Drug: Intervention A Placebo Drug: Intervention B Placebo |
Post Traumatic Stress Disorder | U.S. Army Medical Research and Development Command |
November 2, 2023 | Phase 2 |
| NCT02015546 | Completed | Drug: Vilazodone | Major Depressive Disorder (MDD) |
Duke University | December 2012 | Phase 3 |
| NCT02436239 | Completed | Drug: Vilazodone | Major Depressive Disorder | Forest Laboratories | May 2, 2015 | Phase 3 |
| NCT01828515 | Completed | Drug: Vilazodone Drug: Placebo |
Memory Impairment | University of Texas Southwestern Medical Center |
December 2012 | Phase 2 |
|
|