| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|
| 靶点 |
H+/K+-ATPase (IC50 = 19 nM)
|
|---|---|
| 体外研究 (In Vitro) |
Vonoprazan(0.1 nM-10 μM;30 分钟)以浓度依赖性方式激活猪胃 H+、K+-ATP 酶 [2]。 Vonoprazan 不会阻断 Na+,K+-ATPase 活性,即使剂量比胃 H+,K+-ATPase 活性的 IC50 值大 500 倍也是如此 [2]。
|
| 体内研究 (In Vivo) |
大鼠的基线和 2-脱氧-D-葡萄糖(200 mg/kg;皮下注射)刺激的胃酸产生完全被 4 mg/kg 剂量的沃诺拉赞(1-4 mg/kg;口服)抑制[2] 。
|
| 酶活实验 |
质子钾腺苷三磷酸酶(H+,K+-ATP酶)抑制活性试验[1]
根据Wallmark等人的方法,从猪的胃中制备了胃粘膜微粒体部分。首先,取出胃,用自来水清洗,浸入3mol/L盐水中,用纸巾擦拭粘膜表面。使用polytron(Kinematica)将胃粘膜分离、切碎,并在含有1 mmol/L EDTA和10 mmol/L三盐酸的0.25 mol/L蔗糖溶液(pH 6.8)中均质化。将获得的匀浆在20000g下离心30分钟,上清液在100000g下离心90分钟。将沉淀物悬浮在0.25mol/L的蔗糖溶液中,叠加在含有7.5%Ficoll的0.25mol/L蔗糖溶液上,在100000g的条件下离心5小时。回收含有两层之间界面的部分,并用0.25mol/L的甘蔗溶液离心洗涤。将获得的微粒体部分用作质子、腺苷三磷酸酶钾标准品。向40μL含有2.5μg/mL(基于蛋白质浓度)酶标准产物的50 mmol/L HEPES Tris缓冲液(5 mmol/L氯化镁、10 mmol/L氯化钾、10μmol/L缬氨酸霉素,pH 6.5)中加入溶解在10%二甲亚砜水溶液中的试验化合物(5μL),并将混合物在37°C下孵育30分钟。通过加入5μL 2 mmol/L三磷酸腺苷Tris盐溶液(50 mmol/L HEPES-Tris缓冲液(5mmol/L氯化镁,pH6.5))开始酶反应。酶反应在37°C下进行20分钟,加入15μL孔雀石绿溶液(0.12%孔雀石绿硫酸溶液(2.5 mol/L)、7.5%钼酸铵和11%吐温20以100:25:2的比例混合)以淬灭反应。将混合物在室温下静置15分钟后,在610nm波长下比色测定无机磷与孔雀石绿的反应产物。此外,以相同的方式测量不含氯化钾的反应溶液中无机磷酸的量,在氯化钾存在下从无机磷酸量中减去该量,以确定H+,K+-ATP酶活性。根据对照的活性值和不同浓度试验化合物的活性值确定抑制率(%),并确定H+,K+-ATP酶活性的50%抑制浓度(IC50)。 |
| 动物实验 |
Animal/Disease Models: Male 7- or 8weeks old SD (Sprague-Dawley) rat[2]
Doses: 0.5 mg/kg, 1 mg/kg, 2 mg/kg, 4 mg/kg Route of Administration: Oral administration Experimental Results: Inhibited basal gastric acid secretion in a dose-dependent manner. Inhibiton of Histamine-Stimulated Acid Secretion in Anesthetized Rats (iv) [1] Seven-week-old male Jcl:Sprague Dawley (SD) rats were used. The animals were fasted for 24 h but had free access to water before the experiment. The pylorus was ligated after anesthetization with urethane (1.2 g/kg, ip), and the abdomen was closed. Drugs and the vehicle were given intravenously just after the pylorus ligation. Three minutes later, histamine·2HCl (30 mg/kg per 10 mL) was injected subcutaneously. Three hours after histamine administration, the rats were sacrificed by CO2 asphyxiation and the stomachs were removed. The gastric contents were collected and centrifuged at 3000 rpm for 10 min. The volume of each sample was measured and the acid concentration was determined by automatic titration to pH 7.0 with 0.1 mol/L NaOH, and the total acid output during the 3 h period (μequiv/(3 h)) was calculated. Histamine-Stimulated Acid Secretion in Heidenhain Pouch Dogs [1] Drugs and the vehicle were given orally (0.2 mL/kg) to the dogs in a blind manner. Histamine·2HCl (30 μg/kg) was injected subcutaneously 1 day before and 1, 3, 6, 24, and 48 h after drugs and the vehicle administration. The gastric juice from the pouch was collected continuously for three consecutive 30 min periods after each dosing with histamine·2HCl. The volume of gastric juice was measured, and the acid concentration was determined by automatic titration to pH 7.0 with 0.1 mol/L NaOH solution. The total acid output during the 90 min period (μequiv/(90 min)) from each time was calculated and expressed as a percentage of the predosing value measured 1 day before the administration. |
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation No information is available on the clinical use of vonoprazan during breastfeeding. Because of liver damage that occurred in nursing rodents, the manufacturer recommends that nursing mothers should pump and discard human milk while taking and for 2 days after the last dose. An alternate drug may be preferred. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding In healthy subjects, the plasma protein binding of vonoprazan ranges from 85% to 88%. At plasma concentrations between 0.1 and 10 mcg/mL, the plasma protein binding of vonoprazan is independent of concentration. |
| 参考文献 |
|
| 其他信息 |
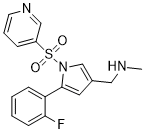
Vonoprazan Fumarate is the fumarate salt form of vonoprazan, a pyrrole derivative and reversible potassium-competitive acid blocker (P-CAB), with potential antacid activity. Upon administration, vonoprazan specifically and competitively binds to the gastric hydrogen-potassium ATPase (H+/K+ ATPase) proton pump at or, more likely, near its potassium ion (K+) binding site and sterically inhibits K+ binding. This blocks the activation of the H+/K+ ATPase by K+, inhibits the proton pump and prevents gastric acid secretion, thereby lowering gastric acid levels.
See also: Vonoprazan (has active moiety); Amoxicillin; clarithromycin; vonoprazan fumarate (component of); Amoxicillin; vonoprazan fumarate (component of). In our pursuit of developing a novel and potent potassium-competitive acid blocker (P-CAB), we synthesized pyrrole derivatives focusing on compounds with low log D and high ligand-lipophilicity efficiency (LLE) values. Among the compounds synthesized, the compound 13e exhibited potent H(+),K(+)-ATPase inhibitory activity and potent gastric acid secretion inhibitory action in vivo. Its maximum efficacy was more potent and its duration of action was much longer than those of proton pump inhibitors (PPIs). Therefore, compound 13e (1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine fumarate, TAK-438) was selected as a drug candidate for the treatment of gastroesophageal reflux disease (GERD), peptic ulcer, and other acid-related diseases. [1] |
| 分子式 |
C17H16FN3O2S
|
|---|---|
| 分子量 |
345.3912
|
| 精确质量 |
345.094
|
| 元素分析 |
C, 59.12; H, 4.67; F, 5.50; N, 12.17; O, 9.26; S, 9.28
|
| CAS号 |
881681-00-1
|
| 相关CAS号 |
Vonoprazan Fumarate;881681-01-2;Vonoprazan hydrochloride;1957202-44-6
|
| PubChem CID |
45375887
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
530.3±60.0 °C at 760 mmHg
|
| 闪点 |
274.5±32.9 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.622
|
| LogP |
2.74
|
| tPSA |
147
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
9
|
| 可旋转键数目(RBC) |
7
|
| 重原子数目 |
32
|
| 分子复杂度/Complexity |
629
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CNCC1=CN(C(=C1)C2=CC=CC=C2F)S(=O)(=O)C3=CN=CC=C3.C(=C/C(=O)O)\C(=O)O
|
| InChi Key |
ROGSHYHKHPCCJW-WLHGVMLRSA-N
|
| InChi Code |
InChI=1S/C17H16FN3O2S.C4H4O4/c1-19-10-13-9-17(15-6-2-3-7-16(15)18)21(12-13)24(22,23)14-5-4-8-20-11-14;5-3(6)1-2-4(7)8/h2-9,11-12,19H,10H2,1H3;1-2H,(H,5,6)(H,7,8)/b;2-1+
|
| 化学名 |
(E)-but-2-enedioic acid;1-[5-(2-fluorophenyl)-1-pyridin-3-ylsulfonylpyrrol-3-yl]-N-methylmethanamine
|
| 别名 |
Vonoprazan; 881681-00-1; TAK-438 free base; Vonoprazan [INN]; Vonoprazan free base; UNII-1R5L3J156G; Vonoprazan [USAN]; 1R5L3J156G;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~289.53 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.24 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.24 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (7.24 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8953 mL | 14.4764 mL | 28.9528 mL | |
| 5 mM | 0.5791 mL | 2.8953 mL | 5.7906 mL | |
| 10 mM | 0.2895 mL | 1.4476 mL | 2.8953 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。