| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
Mutant Isocitrate Dehydrogenase 1 (mIDH1, e.g., R132H mutation) (IC50 = 10 nM for recombinant human mIDH1 R132H) [2]
- Mutant Isocitrate Dehydrogenase 2 (mIDH2, e.g., R140Q, R172K mutations) (IC50 = 14 nM for mIDH2 R140Q; IC50 = 12 nM for mIDH2 R172K) [2] - No significant inhibition of wild-type IDH1/2 (wtIDH1/2) with IC50 > 10 μM, showing >1000-fold selectivity for mutant over wild-type enzymes [2] |
|---|---|
| 体外研究 (In Vitro) |
vorasidenib 的 IC50 小于 50 nM,对人胶质母细胞瘤 U-87 MG pLVX-IDH2 R140Q-neo、纤维肉瘤 HT-1080 和神经球 TS603 细胞表现出有效的抗增殖活性 [2]。
Vorasidenib(0.1-100 nM)剂量依赖性抑制表达mIDH1 R132H的HT1080纤维肉瘤细胞中2-羟基戊二酸(2-HG)的产生,2-HG降低的IC50为12 nM [2] - 在表达mIDH2 R140Q的SK-MEL-28黑色素瘤细胞中,Vorasidenib(0.5-50 nM)在20 nM浓度下使2-HG水平降低85%,恢复正常细胞代谢 [2] - Vorasidenib 对mIDH1/2阳性癌细胞系具有抗增殖活性:72小时后,mIDH1 R132H HT1080细胞GI50 = 25 nM,mIDH2 R140Q SK-MEL-28细胞GI50 = 30 nM;对wtIDH1/2表达细胞无明显作用(GI50 > 5 μM)[2] - Vorasidenib(20 nM)诱导mIDH1 R132H阳性原发性急性髓系白血病(AML)原始细胞分化,髓系分化标志物(CD11b、CD14)表达增加可证实这一效果 [2] |
| 体内研究 (In Vivo) |
AG-881完全穿透血脑屏障。 AG-881 已经开发出来,目前正处于针对 IDH 突变阳性血液恶性肿瘤和实体瘤(包括神经胶质瘤)患者的早期 I 期测试。
荷mIDH1 R132H HT1080异种移植瘤的裸鼠接受Vorasidenib(50 mg/kg,灌胃,每日1次,连续21天)处理。肿瘤组织2-HG水平降低90%,肿瘤生长抑制率达65% [2] - 在患者来源mIDH2 R140Q AML异种移植瘤(PDX)模型中,Vorasidenib(40 mg/kg,灌胃,每日1次×28天)使骨髓2-HG浓度降低88%,原始细胞浸润减少60% [2] |
| 动物实验 |
In pharmacological studies, Vorasidenib exhibited excellent brain penetration and dose-dependently reduced D-2-HG levels. In pharmacokinetics studies, Vorasidenib showed rapid oral absorption and relatively low total body plasma clearance in mice (0.406 L h–1 kg–1) and rats (0.289 L h–1 kg–1). Recently, Vorasidenib entered a phase I clinical trial in patients with advanced solid tumors to investigate its PK/PD, safety, and clinical activity (NCT02481154). Another phase I clinical trial is focusing on patients with mIDH1/2 advanced hematologic cancers (NCT02492737). Excitingly, a phase I study of Vorasidenib and 4 in glioma will soon begin to evaluate the suppression of 2-HG in IDH1 mutant gliomas in resected tumor tissue after presurgical treatment with Vorasidenib or 4 (NCT03343197). [2]
|
| 药代性质 (ADME/PK) |
Absorption
Vorasidenib maximum plasma concentration (Cmax) and AUC increased approximately proportionally over the dose range of 10 to 200 mg (0.2 to 4 times the exposure of the highest approved recommended dosage) following once-daily administration of single and multiple doses. At the highest approved recommended dosage, steady-state mean (CV%) Cmax is 133 ng/mL (73%) and AUC is 1,988 h x ng/mL (95%). A steady state is achieved within 28 days of once-daily dosing, and the mean accumulation ratio of AUC is 4.4. The median (minimum, maximum) time to maximum plasma concentrations (Tmax) at steady-state is 2 hours (0.5 to 4 hours). The mean absolute bioavailability of vorasidenib is 34%. A high-fat and high-calorie (total 800-1,000 calories, of which 500-600 from fat) meal increased vorasidenib Cmax 3.1-fold and AUC 1.4-fold, compared to the fasting conditions. A low-fat and low-calorie (total 400-500 calories, of which 100-125 from fat) meal increased vorasidenib Cmax 2.3-fold and AUC 1.4-fold, compared to the fasting conditions. Route of Elimination Following a single oral radiolabeled dose of vorasidenib, 85% of the dose was recovered in feces (56% unchanged) and 4.5% was recovered in urine. Volume of Distribution The mean (CV%) volume of distribution at steady-state of vorasidenib is 3,930 L (40%). Vorasidenib penetrates the blood-brain barrier: The brain tumour-to-plasma concentration ratio is 1.6. Clearance The mean (CV%) steady state oral clearance is 14 L/h (56%). Protein Binding The protein binding is 97% in human plasma independent of vorasidenib concentrations in vitro. Metabolism / Metabolites Vorasidenib is primarily metabolized by CYP1A2 with minor contributions from CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A. Non-CYP pathways may contribute up to 30% of its metabolism. The exact metabolic pathways and metabolites have not been fully elucidated. Biological Half-Life The mean (CV%) steady state terminal half-life is 10 days (57%). |
| 毒性/毒理 (Toxicokinetics/TK) |
The most common (≥15%) adverse reactions were fatigue, headache, COVID-19 infection, musculoskeletal pain, diarrhea, nausea, and seizure. The most common Grade 3 or 4 laboratory abnormalities (>2%) were increased alanine aminotransferase, increased aspartate aminotransferase, GGT increased, and decreased neutrophils.
|
| 参考文献 | |
| 其他信息 |
Vorasidenib is an orally available inhibitor of mutated forms of both isocitrate dehydrogenase type 1 (IDH1, IDH1 [NADP+] soluble) in the cytoplasm and type 2 (IDH2, isocitrate dehydrogenase [NADP+], mitochondrial) in the mitochondria, with potential antineoplastic activity. Upon administration, vorasidenib specifically inhibits mutant forms of IDH1 and IDH2, thereby inhibiting the formation of the oncometabolite 2-hydroxyglutarate (2HG) from alpha-ketoglutarate (a-KG). This prevents 2HG-mediated signaling and leads to both an induction of cellular differentiation and an inhibition of cellular proliferation in tumor cells expressing IDH mutations. In addition, vorasidenib is able to penetrate the blood-brain barrier (BBB). IDH1 and 2, metabolic enzymes that catalyze the conversion of isocitrate into a-KG, play key roles in energy production and are mutated in a variety of cancer cell types. In addition, mutant forms of IDH1 and 2 catalyze the formation of 2HG and drive cancer growth by blocking cellular differentiation and inducing cellular proliferation.
Vorasidenib (AG-881) is an oral, potent, selective dual inhibitor of mutant IDH1 and IDH2 enzymes [1][2] - Its mechanism of action involves binding to the allosteric site of mIDH1/2, blocking the aberrant conversion of isocitrate to 2-HG. Reduced 2-HG accumulation reverses epigenetic dysregulation (e.g., histone and DNA hypermethylation) and restores normal cell differentiation [1][2] - The drug is being developed for the treatment of cancers harboring mIDH1/2 mutations, including acute myeloid leukemia (AML), low-grade gliomas (LGG), and cholangiocarcinoma [1][2] - It exhibits favorable selectivity for mutant IDH enzymes over wild-type counterparts, minimizing off-target effects on normal cellular metabolism [2] - Preclinical data support its potential as a targeted therapy for mIDH1/2-driven malignancies, with advancing clinical trials evaluating efficacy and safety in patients [1][2] Vorasidenib is a first-in-class dual isocitrate dehydrogenase-1 (IDH1) and isocitrate dehydrogenase-2 (IDH2) inhibitor. It works by suppressing the levels of D-2-hydroxyglutarate (2-HG), an oncometabolite produced by mutant IDH1 and IDH2 isoforms. Vorasidenib displayed improved brain penetration and higher drug exposure compared to other IDH inhibitors such as [ivosidenib] and [enasidenib]. Vorasidenib was first approved by the FDA on August 6, 2024, for the treatment of Grade 2 astrocytoma or oligodendroglioma with a susceptible IDH1 or IDH2 mutation. Vorasidenib is an Isocitrate Dehydrogenase 1 Inhibitor and Isocitrate Dehydrogenase 2 Inhibitor. The mechanism of action of vorasidenib is as an Isocitrate Dehydrogenase 1 Inhibitor and Isocitrate Dehydrogenase 2 Inhibitor and Cytochrome P450 3A Inducer. VORASIDENIB is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2024 and is indicated for astrocytoma and has 4 investigational indications. Vorasidenib is indicated for the treatment of adult and pediatric patients 12 years and older with Grade 2 astrocytoma or oligodendroglioma with a susceptible isocitrate dehydrogenase-1 (IDH1) or isocitrate dehydrogenase-2 (IDH2) mutation following surgery including biopsy, sub-total resection, or gross total resection. Vorasidenib works to reduce tumour growth and invasion in IDH-mutant glioma. In patients with low-grade IDH-mutant glioma, vorasidenib significantly improved progression-free survival and delayed the time to the next anticancer intervention. Vorasidenib decreases 2-HG tumour concentrations in patients with IDH1 or IDH2 mutated glioma. Relative to tumours from patients in the untreated group, the posterior median percentage reduction (95% credible interval) in tumour 2-HG was 64% (22%, 88%) to 93% (76%, 98%) in tumours from patients who received vorasidenib at exposures that were 0.3 to 0.8 times the exposure observed with the highest recommended dosage. The exposure-response relationship and time course of pharmacodynamic response for the safety and effectiveness of vorasidenib have not been fully characterized. Mutations in the isocitrate dehydrogenase 1 and 2 (IDH1/2) enzymes can be identified in various malignancies, including acute myeloid leukemia (AML) and gliomas. Mutant enzymes produce an oncometabolite D-2-hydroxyglutarate (2-HG), which contributes to oncogenesis and tumour growth by blocking the activity of α-ketoglutarate–dependent enzymes and causing epigenetic dysregulation, such as global DNA hypermethylation, and interfering with immunity. Vorasidenib is a small molecule inhibitor that targets isocitrate dehydrogenase-1 and 2 (IDH1 and IDH2) enzymes. In vitro, vorasidenib inhibited the IDH1 wild-type and mutant variants, including R132H and the IDH2 wild-type and mutant variants. In cell-based and in vivo tumour models expressing IDH1 or IDH2 mutated proteins, vorasidenib decreased the production of 2-2-HG and partially restored cellular differentiation. Isocitrate dehydrogenase (IDH) is an essential enzyme for cellular respiration in the tricarboxylic acid (TCA) cycle. Recurrent mutations in IDH1 or IDH2 are prevalent in several cancers including glioma, acute myeloid leukemia (AML), cholangiocarcinoma and chondrosarcoma. The mutated IDH1 and IDH2 proteins have a gain-of-function, neomorphic activity, catalyzing the reduction of α-ketoglutarate (α-KG) to 2-hydroxyglutarate (2-HG) by NADPH. Cancer-associated IDH mutations block normal cellular differentiation and promote tumorigenesis via the abnormal production of the oncometabolite 2-HG. High levels of 2-HG have been shown to inhibit α-KG dependent dioxygenases, including histone and deoxyribonucleic acid (DNA) demethylases, which play a key role in regulating the epigenetic state of cells. Current targeted inhibitors of IDH1 (AG120, IDH305), IDH2 (AG221), and pan-IDH1/2 (AG881) selectively inhibit mutant IDH protein and induce cell differentiation in in vitro and in vivo models. Preliminary results from phase I clinical trials with IDH inhibitors in patients with advanced hematologic malignancies have demonstrated an objective response rate ranging from 31% to 40% with durable responses (>1 year) observed. Furthermore, the IDH inhibitors have demonstrated early signals of activity in solid tumors with IDH mutations, including cholangiocarcinomas and low grade gliomas.[1] Isocitrate dehydrogenases 1 and 2 (IDH1/2) are homodimeric enzymes that catalyze the conversion of isocitrate to α-ketoglutarate (α-KG) in the tricarboxylic acid cycle. However, mutant IDH1/2 (mIDH1/2) reduces α-KG to the oncometabolite 2-hydroxyglutarate (2-HG). High levels of 2-HG competitively inhibit the α-KG-dependent dioxygenases involved in histone and DNA demethylation, thereby impairing normal cellular differentiation and promoting tumor development. Thus, small molecules that inhibit these mutant enzymes may be therapeutically beneficial. Recently, an increasing number of mIDH1/2 inhibitors have been reported. In this review, we summarize the molecular basis of mIDH1/2 and the activity, binding modes, and progress in clinical application of mIDH1/2 inhibitors. We note important future research directions for mIDH1/2 inhibitors and discuss potential therapeutic strategies for the development of mIDH1/2 inhibitors to treat IDH1/2 mutated tumors. [2] |
| 分子式 |
C14H13CLF6N6
|
|
|---|---|---|
| 分子量 |
414.7366
|
|
| 精确质量 |
414.079
|
|
| 元素分析 |
C, 40.54; H, 3.16; Cl, 8.55; F, 27.48; N, 20.26
|
|
| CAS号 |
1644545-52-7
|
|
| 相关CAS号 |
2316810-02-1 (citrate)
|
|
| PubChem CID |
117817422
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| LogP |
5.3
|
|
| tPSA |
75.6
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
12
|
|
| 可旋转键数目(RBC) |
5
|
|
| 重原子数目 |
27
|
|
| 分子复杂度/Complexity |
448
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
ClC1=CC=CC(C2=NC(=NC(=N2)N[C@H](C)C(F)(F)F)N[C@H](C)C(F)(F)F)=N1
|
|
| InChi Key |
QCZAWDGAVJMPTA-RNFRBKRXSA-N
|
|
| InChi Code |
InChI=1S/C14H13ClF6N6/c1-6(13(16,17)18)22-11-25-10(8-4-3-5-9(15)24-8)26-12(27-11)23-7(2)14(19,20)21/h3-7H,1-2H3,(H2,22,23,25,26,27)/t6-,7-/m1/s1
|
|
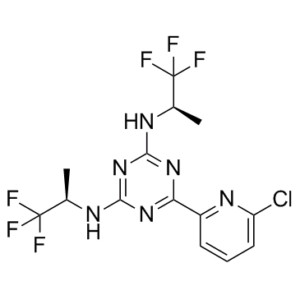
| 化学名 |
6-(6-chloropyridin-2-yl)-2-N,4-N-bis[(2R)-1,1,1-trifluoropropan-2-yl]-1,3,5-triazine-2,4-diamine
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (6.03 mM) in 5% DMSO + 40% PEG300 + 5% Tween80 + 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
*生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.4 mg/mL (5.79 mM) in 5% DMSO + 95% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: 2.08 mg/mL (5.02 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 配方 4 中的溶解度: 2.08 mg/mL (5.02 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将100μL 20.8mg/mL澄清的DMSO储备液加入到900μL 20%SBE-β-CD生理盐水中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 5 中的溶解度: 2.08 mg/mL (5.02 mM) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4111 mL | 12.0557 mL | 24.1115 mL | |
| 5 mM | 0.4822 mL | 2.4111 mL | 4.8223 mL | |
| 10 mM | 0.2411 mL | 1.2056 mL | 2.4111 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05592743 | AVAILABLE | Drug: Vorasidenib | Disease Attributes Glioma Neoplasms |
Servier | ||
| NCT05843708 | RECRUITING | Drug:Vorasidenib 40 mg Oral Tablet Drug:Ciprofloxacin 500 mg Oral Tablet Drug:Vorasidenib 10 mg Oral Tablet |
Healthy Subjects | Servier Bio-Innovation LLC | 2023-04-14 | Phase 1 |
| NCT05484622 | RECRUITING | Drug: Vorasidenib Drug: Pembrolizumab |
Astrocytoma | Institut de Recherches Internationales Servier |
2023-01-20 | Phase 1 |
| NCT05609994 | NOT YET RECRUITING | Drug: PEPIDH1M vaccine+vorasidenib |
Low Grade Glioma of Brain | Katy Peters, MD, PhD | 2024-06 | Phase 1 |
| NCT04164901 | ACTIVE | Drug: Vorasidenib Drug: Matching Placebo |
Grade 2 Glioma Recurrent Glioma Residual Glioma |
Institut de Recherches Internationales Servier |
2020-01-05 | Phase 3 |