| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| 5g |
|
||
| 10g |
|
||
| Other Sizes |
|

| 靶点 |
HIV reverse transcriptase (NRTI)
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
zidovudine 的 EC50 值分别为 17、1311、8 和 5 nM,可抑制 SVG、原代人胎儿星形胶质细胞 (PFA)、外周血单核细胞 (PBMC) 和单核细胞源性巨噬细胞 (MDM)。齐多夫定的 EC90 值分别为 0.205 μM、44.157 μM、0.481 μM 和 0.219 μM,抑制 SVG、PFA、PBMC 和 MDM [1]。 CRISPR/Cas9 基因组编辑已成为一种可靠且高效的技术,可精确改变活细胞中基因组的特定区域。 CXCR4 被认为是艾滋病的关键治疗靶点,因为它作为 HIV-1 感染的辅助受体发挥作用。通过将自身附着在包膜蛋白 gp120 上,CXCR4 有助于病毒进入人类 CD4+ 细胞。 CRISPR/Cas9 介导的基因组编辑有效破坏人类 CXCR4 基因,导致人类原代 CD4+ T 细胞产生 HIV-1 耐药性。 Cas9 介导的 CXCR4 消融表现出高特异性和最小的脱靶效应,对细胞分裂或增殖没有影响 [2]。
|
||
| 体内研究 (In Vivo) |
在野生型小鼠中,与 PBS 载体相比,NRTI 拉米夫定 (3TC)、齐多夫定 (AZT) 或阿巴卡韦 (ABC) 抑制激光诱导的脉络膜新生血管 (CNV)。激光损伤后第 3 天,RPE/脉络膜中的平均 VEGF-A 水平达到峰值。用 3TC、AZT 和 ABC 处理的小鼠眼睛中这种蛋白质的水平显着低于野生型小鼠的对照眼睛,但 P2rx7-/- 小鼠的眼睛则不然。 [3]。
|
||
| 酶活实验 |
Env假型萤光素酶报告病毒的产生和定量[1]
如前所述,通过使用Lipofectamine 2000(Invitrogen)以1∶3∶1的比例用pCMVΔP1ΔenvpA、pHIV-1Luc和pcDNA3-VSVg或pSVIII-YU2-Env质粒转染293T细胞,产生Env假型萤光素酶报告病毒。使用HIV-1 YU-2包膜糖蛋白用CCR5假型的病毒用于PBMC和MDM的感染,而SVG细胞和PFA用水泡性口腔炎病毒G蛋白(VSV-G)假型病毒感染,以获得足够水平的病毒进入用于抑制测定。48小时后采集含有假病毒类型的上清液,通过0.45µm过滤器过滤,在每种不同的细胞类型上滴定(计算TCID50值),并在−80°C下储存。 |
||
| 细胞实验 |
细胞活力测定[1]
根据制造商的方案,使用CellTitre-Glo发光细胞活力测定法(Promega,USA)在药物暴露后72小时评估所有细胞类型的ARV细胞毒性。[1] 病毒抑制试验[1] 在存在滴定浓度的抗逆转录病毒药物的情况下,对所有细胞类型进行分析。将5000个SVG、2500个PFA、200000个PBMC或50000个MDM细胞/孔接种到96孔板的一式三孔中。24小时后,取出培养基,用含有ARV或DMSO(0.5%vol/vol)的培养基代替,并将相当的TCID50感染单位的荧光素酶报告病毒添加到细胞中。在37°C下孵育16小时后,去除初始病毒接种物,并用含有相同ARV或DMSO(0.5%体积/体积)浓度的培养基代替。在感染后72小时,抽吸培养基,裂解细胞,并根据制造商的说明使用萤光素酶测定系统(Promega)测量HIV-1感染。使用FLUOStar Optima微孔板读取器(BMG Labtech,德国)测量发光。抑制曲线以及50%(EC50)和90%(EC90)的有效浓度通过如前所述的非线性回归分析使用GraphPad Prism软件确定。[1] |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Rapid and nearly complete absorption from the gastrointestinal tract following oral administration; however, because of first-pass metabolism, systemic bioavailability of zidovudine capsules and solution is approximately 65% (range, 52 to 75%). Bioavailability in neonates up to 14 days of age is approximately 89%, and it decreases to approximately 61% and 65% in neonates over 14 days of age and children 3 months to 12 years, respectively. Administration with a high-fat meal may decrease the rate and extent of absorption. As in adult patients, the major route of elimination was by metabolism to GZDV. After intravenous dosing, about 29% of the dose was excreted in the urine unchanged and about 45% of the dose was excreted as GZDV. Apparent volume of distribution, HIV-infected patients, IV administration = 1.6 ± 0.6 L/kg 0.65 +/- 0.29 L/hr/kg [HIV-infected, Birth to 14 Days of Age] 1.14 +/- 0.24 L/hr/kg [HIV-infected, 14 Days to 3 Months of Age] 1.85 +/- 0.47 L/hr/kg [HIV-infected, 3 Months to 12 Years of Age]. The transporters, ABCB1, ABCC4, ABCC5, and ABCG2 are involved with the clearance of zidovudine. In patients with impaired renal function, plasma concentrations of zidovudine may be increased and the half-life prolonged. In one study in adults with impaired renal function (creatinine clearances ranging from 6-31 ml/minute) without HIV infections beta half life of zidovudine averaged 1.4 hours and was similar to that reported for adults with HIV infections who had normal renal function. However, the beta half life of glucuronide in these adults with impaired renal function averaged 8 hours and was considerably prolonged compared with that reported for adults with HIV infections who had normal renal function. In one study in adults with hemophilia and HIV infections who had elevated serum concentrations of aspartate aminotransferase (serum glutamic-oxaloacetic transaminase), alanine aminotransferase (serum glutamic-pyruvic transaminase), pharmacokinetics of zidovudine after a single 300 mg oral dose showed considerable interindividual variation. Following oral administration of zidovudine in patients with HIV infections, 63-95% of the dose is excreted in urine; approximately 14-18% of the dose is excreted as unchanged zidovudine and 72-74% is excreted as zidovudine 5'-O-glucuronide within 6 hours. Following iv administration of the drug in adults or children with HIV infections, approximately 18-29% of the dose is excreted in urine as unchanged drug and 45-60% is excreted as zidovudine 5'-O-glucuronide within 6 hours. Zidovudine and 3'-azodp-3'-deoxy-5'-O-beta-d-glucopyranuronosylthymidine are eliminated principally in urine via both glomerular filtration and tubular secretion. Following oral or IV administration in adults with HIV infection, total body clearance of zidovudine averages 1.6 l/hr per kg (range: 0.8-2.7 l/hr per kg) and renal clearance of the drug averages 0.34 l/hr per kg. In children 3 months to 12 years of age, the total body clearance averaged 1.85 l/hr per kg. In one limited study in neonates and infants younger than 3 months of age, total body clearance of the drug averaged 0.65 l/hr per kg in those 14 days of age or younger and 1.14 l/hr per kg in those older than 14 days of age. Zidovudine is rapidly metabolized via glucuronidation in the liver to zidovudine 5'-O-glucuronide (GAZT); the metabolite has an apparent elimination half life of 1 hour (range: 0.6-1.7 hours). Zidovudine 5'-O-glucuronide does not appear to have antiviral activity against HIV. For more Absorption, Distribution and Excretion (Complete) data for ZIDOVUDINE (21 total), please visit the HSDB record page. Metabolism / Metabolites Hepatic. Metabolized by glucuronide conjugation to major, inactive metabolite, 3′-azido-3′-deoxy-5′- O-beta-D-glucopyranuronosylthymidine (GZDV). UGT2B7 is the primary UGT isoform that is responsible for glucuronidation. Compared to zidovudine, GZDV's area under the curve is approximately 3-fold greater. The cytochrome P450 isozymes are responsible for the reduction of the azido moiety to form 3'-amino-3'- deoxythymidine (AMT). Zidovudine is rapidly metabolized via glucuronidation in the liver principally to 3-azido-3-deoxy-5-O-beta-d-glucopyranuronosylthymidine (GZDV; formerly GAZT); zidovudine is also metabolized to GZDV in renal microsomes. GZDV has an apparent elimination half-life of 1 hour (range: 0.6-1.7 hours) and does not appear to have antiviral activity against HIV. In addition, two other hepatic metabolites of zidovudine have been identified as 3-amino-3-deoxythymidine (AMT) and its glucuronide derivative (GAMT). Intracellularly, in both virus-infected and uninfected cells, zidovudine is converted to zidovudine monophosphate by cellular thymidine kinase; the monophosphate derivative is phosphorylated to zidovudine diphosphate via cellular dTMP kinase (thymidylate kinase) and then to zidovudine triphosphate via other cellular enzymes. Intracellular (host cell) conversion of zidovudine to the triphosphate derivative is necessary for the antiviral activity of the drug. Activation for antibacterial action, however, does not depend on phosphorylation within host cells but rather depends on conversion within bacterial cells. The mechanisms of intestinal mucosal transport and metabolism of zidovudine and other thymidine analogs were studied. No zidovudine metabolites appeared in any part of the gastrointestinal tract. Other thymidine analogs were rapidly metabolized in the upper gastrointestinal tract, but not in the colon. Hepatic. Metabolized by glucuronide conjugation to major, inactive metabolite, 3′-azido-3′-deoxy-5′- O-beta-D-glucopyranuronosylthymidine (GZDV). UGT2B7 is the primary UGT isoform that is responsible for glucuronidation. Compared to zidovudine, GZDV's area under the curve is approximately 3-fold greater. The cytochrome P450 isozymes are responsible for the reduction of the azido moiety to form 3'-amino-3'- deoxythymidine (AMT). Route of Elimination: As in adult patients, the major route of elimination was by metabolism to GZDV. After intravenous dosing, about 29% of the dose was excreted in the urine unchanged and about 45% of the dose was excreted as GZDV. Half Life: Elimination half life, HIV-infected patients, IV administration = 1.1 hours (range of 0.5 - 2.9 hours) Biological Half-Life Elimination half life, HIV-infected patients, IV administration = 1.1 hours (range of 0.5 - 2.9 hours) The plasma half-life of zidovudine in adults averages approximately 0.53 hours following oral or IV administration. Following IV administration of zidovudine in adults or children, plasma concentrations of the drug appear to decline in a biphasic manner. Half-life in adults is less than 10 minutes in the initial phase and 1 hour in the terminal phase. Following IV administration over 1 hour of a single 80-, 120-, or 160-mg/sq m , dose in children 1-13 years of age with symptomatic HIV infection, the alpha half-life of zidovudine averaged 0.16-0.25 hours and the beta half-life averaged 1-1.7 hours. Plasma half-life of zidovudine generally is longer in neonates than in older children and adults but decreases with neonatal maturity. In one limited study in neonates and infants younger than 3 months of age, plasma half-life of zidovudine averaged 3.1 hours in those 14 days of age or younger and 1.9 hours in those older than 14 days of age. In a study in premature neonates (26-32 weeks gestation; birthweight 0.7-1.9 kg), the serum half-life of zidovudine averaged 7.3 hours at an average postnatal age of 6.3 days and averaged 4.4 hours at an average postnatal age of 17.7 days. The value for half-life of zidovudine is 1-2 hr. |
||
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
Zidovudine, a structural analog of thymidine, is a prodrug that must be phosphorylated to its active 5ду_-triphosphate metabolite, zidovudine triphosphate (ZDV-TP). It inhibits the activity of HIV-1 reverse transcriptase (RT) via DNA chain termination after incorporation of the nucleotide analogue. It competes with the natural substrate dGTP and incorporates itself into viral DNA. It is also a weak inhibitor of cellular DNA polymerase ‘± and ‘_. Toxicity Data Male rat(po): LD50 = 3.1 g/kg Female rat(po): LD50 = 3.7 g/kg Male mice: LD50 = 3.6 g/kg Female mice(po): LD50 = 3.1 g/kg LD50 is 3084 mg/kg (orally in mice). Interactions Concomitant use of probenecid may produce substantially higher and prolonged serum concentrations of zidovudine. In at least one study, concomitant use of acetaminophen reportedly resulted in an increased risk of granulocytopenia in patients receiving zidovudine; this potentiation of hematologic toxicity appeared to correlate with the duration of acetaminophen use. Neurotoxicity (profound drowsiness and lethargy), which recurred on rechallenge, has been reported in at least one HIV-infected patient who received acyclovir and zidovudine concomitantly. Neurotoxicity was evident within 30-60 days after initiation of IV acyclovir therapy, persisted with some improvement when acyclovir was administered orally, and resolved following discontinuance of acyclovir in this patient. Acyclovir and zidovudine have been used concomitantly in other HIV-infected patients without evidence of increased toxicity. Although the clinical importance is unclear, there is some evidence that acyclovir may potentiate the antiretroviral effect of zidovudine in vitro; acyclovir alone has only minimal antiretroviral activity. Both ganciclovir and zidovudine alone produce direct, dose-dependent inhibitory effects on myeloid and erythroid progenitor cells, and combined use of the drugs increases the risk of hematologic toxicity and may result in additive or synergistic myelotoxic effects. In several studies in patients with AIDS and cytomegalovirus infections, profound, intolerable myelosuppression, evidenced principally as severe neutropenia, occurred in all patients receiving ganciclovir (5 mg/kg iv 1-4 times daily) concomitantly with zidovudine (200 mg orally every 4 hours); anemia also occurred in many of these patients. Severe hematologic toxicity, which required a reduction in zidovudine dosage, also occurred in more than 80% of patients receiving ganciclovir (5 mg/kg iv 1-2 times daily) concomitantly with zidovudine (100 mg orally every 4 hours). For more Interactions (Complete) data for ZIDOVUDINE (18 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 Rat iv > 750 mg/kg LD50 Mice iv > 3000 mg/kg |
||
| 参考文献 |
|
||
| 其他信息 |
Therapeutic Uses
Anti-HIV Agents; Antimetabolites; Antimetabolites, Antineoplastic; Reverse Transcriptase Inhibitors Zidovudine is indicated in combination with other antiretroviral agents for the treatment of HIV infection. ... /Included in US product labeling/ Zidovudine is indicated for the prevention of mother-to-child transmission of HIV-1 infection as part of a regimen that includes oral zidovudine beginning between 14 and 34 weeks gestation, continuous intravenous infusion of zidovudine during labor, and administration of zidovudine syrup to the neonate for the first 6 weeks of life. However, transmission to infants may still occur in some cases despite the use of this regimen. /Included in US product labeling/ Zidovudine has been used prophylactically in health care workers at risk of acquiring HIV infection after occupational exposure to the virus. Risk of transmission from a single needlestick is approximately 0.3%. Efficacy, and optimal dose and duration of prophylactic treatment are unknown at this time; however, HIV infection has occurred in persons who received zidovudine prophylaxis after a needlestick or other parenteral exposure. /NOT included in US product labeling/ For more Therapeutic Uses (Complete) data for ZIDOVUDINE (7 total), please visit the HSDB record page. Drug Warnings Adverse systemic effects reported with IV zidovudine are similar to those reported with oral zidovudine. However, clinical experience with IV zidovudine has been more limited than experience with oral zidovudine and the drug has generally been administered IV only for short periods of time. Long-term IV zidovudine therapy (i.e., longer than 2-4 weeks) has not been evaluated in adults and may enhance adverse hematologic effects. The most common adverse effects of zidovudine are hematologic effects (i.e., anemia, neutropenia), nausea, and headache. Because HIV-infected patients receiving zidovudine generally have serious underlying disease with multiple baseline symptomatology and clinical abnormalities and because many adverse effects that occurred in zidovudine-treated patients also occurred in patients receiving placebo, many reported effects may not be directly attributable to zidovudine. The frequency and severity of adverse effects associated with use of zidovudine in adults are greater in patients with more advanced disease at the time of initiation of therapy. In one study in asymptomatic patients receiving 100 mg of the drug orally 5 times daily for an average of longer than 1 year (range: 4 months to 2 years), only nausea occurred more frequently in patients receiving zidovudine than in those receiving placebo. Adverse effects reported with use of zidovudine in women, IV drug users, and racial minorities are similar to those reported with use of the drug in white males. Four patients with the acquired immunodeficiency syndrome, and a history of Pneumocystis carinii pneumonia developed severe pancytopenia (hemoglobin, less than 85 g/l; granulocytes, less than or equal to 0.5 X 10(9)/L; platelets, less than or equal to 30 X 10(9)/L) 12 to 17 weeks after the initiation of azidothymidine (AZT) therapy. The bone marrow was markedly hypocellular in three patients and moderately hypocellular in the fourth. Partial bone marrow recovery was documented within 4 to 5 weeks in three patients, but no marrow recovery has yet occurred in one patient during the more than 6 months since AZT treatment was discontinued. Hematologic toxicity is causally related to zidovudine therapy, being directly related to dosage and duration of therapy with the drug, and has been reported most frequently in patients with advanced symptomatic HIV infection or low pretreatment hemoglobin concentrations, neutrophil counts, and helper/inducer (CD4+, T4+) T-cell counts. Patients with low serum folate or vitamin B12 concentrations may be at increased risk for developing bone marrow toxicity during zidovudine therapy. There also are limited data suggesting that bone marrow of patients with fulminant acquired immunodeficiency syndrome (AIDS) may be more sensitive to zidovudine-induced toxicity than that of patients with less advanced disease (e.g., AIDS-related complex (ARC)). For more Drug Warnings (Complete) data for ZIDOVUDINE (43 total), please visit the HSDB record page. Pharmacodynamics Zidovudine is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Zidovudine is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The lack of a 3'-OH group in the incorporated nucleoside analogue prevents the formation of the 5' to 3' phosphodiester linkage essential for DNA chain elongation, and therefore, the viral DNA growth is terminated. |
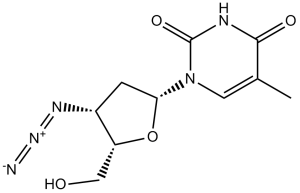
| 分子式 |
C10H13N5O4
|
|
|---|---|---|
| 分子量 |
267.24
|
|
| 精确质量 |
267.096
|
|
| 元素分析 |
C, 44.94; H, 4.90; N, 26.21; O, 23.95
|
|
| CAS号 |
30516-87-1
|
|
| 相关CAS号 |
117675-21-5 (glucuronide); 106060-89-3 (diphosphate); 92586-35-1 (triphosphate)
|
|
| PubChem CID |
35370
|
|
| 外观&性状 |
White to off-white solid
|
|
| 熔点 |
113-115 °C(lit.)
|
|
| 折射率 |
47 ° (C=1, H2O)
|
|
| LogP |
-0.53
|
|
| tPSA |
134.07
|
|
| 氢键供体(HBD)数目 |
2
|
|
| 氢键受体(HBA)数目 |
6
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
19
|
|
| 分子复杂度/Complexity |
484
|
|
| 定义原子立体中心数目 |
3
|
|
| SMILES |
O=C(C(C)=CN1[C@@H](C2)O[C@@H]([C@H]2N=[N+]=[N-])CO)NC1=O
|
|
| InChi Key |
HBOMLICNUCNMMY-XLPZGREQSA-N
|
|
| InChi Code |
InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1
|
|
| 化学名 |
1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (9.35 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (9.35 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (9.35 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 18 mg/mL (67.4 mM) 配方 5 中的溶解度: 20 mg/mL (74.84 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶 (<60°C). 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7420 mL | 18.7098 mL | 37.4195 mL | |
| 5 mM | 0.7484 mL | 3.7420 mL | 7.4839 mL | |
| 10 mM | 0.3742 mL | 1.8710 mL | 3.7420 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Early Infant HIV Treatment in Botswana
CTID: NCT02369406
Phase: Phase 2/Phase 3 Status: Active, not recruiting
Date: 2023-11-09
|
|
|