| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
PKD3 (IC50 = 109.4 nM); PKD2 (IC50 = 133.4 nM); PKD1 (IC50 = 154.6 nM); v-Fyn (IC50 = 0.6 μM); c-Abl (IC50 = 0.6 μM)
Protein Kinase D (PKD) isoforms (PKD1, PKD2, PKD3) (IC₅₀≈100 nM for each isoform) [2] Engineered gatekeeper mutant PKD1(M659G) (12-fold higher sensitivity to 1-Naphthyl PP1 (1-NA-PP1) compared to wild-type PKD1) [2] Analog-sensitive Protein Kinase C epsilon (AS-PKCε) (no definite IC₅₀, Ki, or EC₅₀ data provided; does not inhibit wild-type PKCε) [3] |
|---|---|
| 体外研究 (In Vitro) |
1-NA-PP1和IKK-16是新型泛pkd抑制剂。[1]
1-NA-PP1是一种atp竞争性抑制剂,对密切相关的激酶具有高选择性。[1] 1-NA-PP1在前列腺癌细胞中具有细胞活性并引起靶抑制。[1] 1-NA-PP1通过诱导G2/M阻滞有效阻断前列腺癌细胞增殖。[1] 1- na - pp1诱导的生长停滞是通过靶向抑制PKD介导的。[1] 1-NA-PP1能有效抑制前列腺肿瘤细胞的迁移和侵袭。[1] 在完整细胞中,PKD1的守门人突变体对1-NA-PP1抑制的敏感性提高了12倍。[1] 1. 重组PKD亚型抑制作用:在体外放射学激酶实验中,1-萘基PP1(1-NA-PP1)以浓度依赖性方式抑制重组人PKD1、PKD2和PKD3的活性。所有三种亚型的IC₅₀均约为100 nM,计算方式为至少3次独立实验的均值±标准误,每个抑制剂浓度设3次重复测定[2] 2. ATP竞争性抑制:1-萘基PP1(1-NA-PP1)是PKD1的ATP竞争性抑制剂。通过在递增ATP浓度下测定PKD1活性,并绘制不同1-萘基PP1(1-NA-PP1)浓度对应的双倒数图(Lineweaver-Burke图),观察到竞争性抑制特征性的平行线,证实其作用机制[2] 3. 激酶选择性:1-萘基PP1(1-NA-PP1)对PKD具有高选择性,不抑制相关激酶(包括PKCα、PKCδ(测试浓度10 nM、100 nM、1 μM、10 μM)和CAMKIIα),而阳性对照PKC抑制剂GF109203X可有效抑制PKCα和PKCδ[2] 4. LNCaP细胞中PKD1激活抑制:不同剂量1-萘基PP1(1-NA-PP1)预处理LNCaP细胞45分钟,可阻断10 nM PMA(刺激20分钟)诱导的内源性PKD1在S⁹¹⁶和S⁷⁴⁴/⁷⁴⁸位点的磷酸化(Western blot检测)。对Western blot条带进行光密度定量分析,可计算抑制PKD1激活的IC₅₀[2] 5. PC3细胞抗增殖作用:10 μM 1-萘基PP1(1-NA-PP1)可强效阻断PC3前列腺癌细胞增殖。将PC3细胞以3复孔接种于24孔板,过夜贴壁后第1天计数(基线),加入1-萘基PP1(1-NA-PP1)或溶媒(DMSO),每2天更换培养基和抑制剂,连续5天每日计数,结果显示抑制剂组细胞数量显著低于溶媒组[2] 6. 细胞活力抑制:1-萘基PP1(1-NA-PP1)可诱导PC3细胞死亡。96孔板中接种PC3细胞(3000个细胞/孔),过夜贴壁后加入0.3–100 μM 1-萘基PP1(1-NA-PP1)(溶媒为对照),孵育72小时。加入MTT溶液(5 mg/mL)孵育4小时,弃上清后加DMSO溶解甲臜结晶,在570 nm处测定吸光度。计算相对细胞活力,IC₅₀为2次独立实验的均值[2] 7. G2/M期细胞周期阻滞:10 μM 1-萘基PP1(1-NA-PP1)处理PC3细胞48小时可导致G2/M期阻滞。收集细胞用PBS洗涤,70%乙醇-20°C固定过夜,离心弃乙醇后,用含碘化丙啶(50 μg/mL)和RNase A(100 μg/mL)的PBS重悬,37°C孵育30分钟。流式细胞术分析细胞周期分布,显示G2/M期细胞比例显著高于DMSO对照组(p<0.001)[2] 8. 细胞迁移抑制:30 μM 1-萘基PP1(1-NA-PP1)可阻断PC3细胞迁移。PC3细胞在6孔板中培养至100%汇合,用无菌枪头制造均匀划痕,PBS洗涤去除脱落细胞后立即成像(0小时)。加入含30 μM 1-萘基PP1(1-NA-PP1)或DMSO的培养基,孵育22小时后再次成像。图像分析软件测量划痕面积,计算伤口愈合百分比为(1 -(22小时划痕面积/0小时划痕面积))×100[2] 9. 细胞侵袭抑制:30 μM 1-萘基PP1(1-NA-PP1)可抑制DU145前列腺癌细胞侵袭。Transwell小室用Matrigel包被,37°C孵育1小时成胶,上室接种DU145细胞(5×10⁴个细胞/小室)和含抑制剂或DMSO的培养基,下室加完全培养基。孵育20小时后,棉签去除上室非侵袭细胞,下室侵袭细胞用100%甲醇固定10分钟、0.4%苏木精染色15分钟,随机选取6个视野计数,计算相对于DMSO对照组的侵袭百分比[2] 10. PKD过表达拯救实验:60 mm培养皿接种PC3细胞(5×10⁵个细胞),过夜孵育后用含PKD1(Adv-PKD1)、PKD3(Adv-PKD3)或空载体(Adv-null)的腺病毒以50–100 MOI感染。24小时后消化细胞,96孔板接种3000个细胞/孔(活力实验)或Transwell小室接种5×10⁴个细胞/小室(侵袭实验),用10/30 μM 1-萘基PP1(1-NA-PP1)或DMSO处理72小时(活力)或20小时(侵袭),按前述方法进行MTT或Matrigel侵袭实验。抗PKD1/PKD3抗体Western blot验证PKD过表达[2] 11. HEK293细胞突变PKD1磷酸化检测:用转染试剂将Flag标记的野生型PKD1、PKD1(M659G)或PKD1(M659A)质粒转染HEK293细胞。转染2天后血清饥饿24小时,无血清培养基中用递增浓度1-萘基PP1(1-NA-PP1)预处理45分钟,再用10 nM PMA刺激20分钟。裂解细胞提取蛋白,Western blot检测p-S⁹¹⁶-PKD1、Flag(检测PKD1)和微管蛋白(内参)[2] |
| 体内研究 (In Vivo) |
系统给药1-NA-PP1容易穿过血脑屏障,抑制pkcε介导的磷酸化。1-NA-PP1可逆地降低了AS-PKCε小鼠的乙醇消耗量,但对缺乏AS-PKCε突变的野生型小鼠没有作用。这些结果支持了PKCε催化活性抑制剂作为减少乙醇消耗的策略的开发,并且它们表明as - PKCε小鼠是研究PKCε在行为中的作用的有用工具。[3]
我们培育了一种新的AS-PKCε小鼠系,其ATP结合位点发生点突变,使其对纳米摩尔浓度的PP1类似物1-NA-PP1的抑制高度敏感。系统给药的1-NA-PP1穿过血脑屏障,在脑内达到足够高的浓度来抑制AS-PKCε。1-NA-PP1延长了乙醇的共济失调和催眠作用,减少了AS-PKCε小鼠的乙醇消耗。在缺乏AS-PKCε突变的野生型小鼠中未观察到1-NA-PP1的这些作用。这些结果表明,抑制PKCε催化活性的化合物可能有助于减少乙醇的消耗。 [3] 1-NA-PP1降低AS-PKCε小鼠的乙醇消耗[3] 为了确定1-NA-PP1是否会改变乙醇的消耗,我们对AS-PKCε小鼠进行了连续的两瓶选择饮用程序,其中乙醇浓度从3%上升到6%,最后在8天内上升到10%。小鼠习惯了载体注射并连续三次饮用10%乙醇达到稳定水平后[F(2,34) = 1.474, P = 0.2433;图4A],它们使用受试者内设计给药1-NA-PP1,其中所有动物在不同日期接受载体或1-NA-PP1。1-NA-PP1浓度为20或30mg/kg时,前24 h乙醇消耗量降低[F(2,34) = 10.69;P = 0.0003;图4 b)。这种效应是可逆的,因为乙醇消耗量在用载体或1-NA-PP1处理48小时后相似[F(2,34) = 3.058;P = 0.0601;图4 c)。1-NA-PP1未显著改变乙醇偏好[F(2,34) = 0.9508;P = 0.3965;图4 d)。虽然在30mg/kg时有减少水消耗的趋势,但这种影响在统计学上并不显著[F(2,34) = 1.722;P = 0.1940;图4 e]。 [3] 1-NA-PP1延长AS-PKCε小鼠乙醇中毒[3] 我们之前发现Prkce - / -小鼠由于对乙醇的急性功能耐受性受损而表现出长期的乙醇中毒迹象(Hodge等人,1999年,Wallace等人,2007年)。因此,为了确定抑制PKCε是否会改变乙醇中毒,并测试口服1-NA-PP1是否有效产生表型,我们给AS-PKCε小鼠喂食1-NA-PP1或对照食物和水11天。平均而言,1-NA-PP1组小鼠每天消耗3.00±0.14g 1-NA-PP1食物颗粒,低于对照组(3.65±0.16g/d;P = 0.02)。1-NA-PP1组小鼠的饮水量(2.00±0.01ml)也少于对照组(3.5±0.25ml);P < 0.0001)。然而,尽管在食物和水的摄取量上存在差异,但1- na - pp1喂养的动物(25.5±0.18g)和对照喂养的动物(25.8±0.23g)的体重相似。 1. 血脑屏障穿透:对AS-PKCε敲入小鼠腹腔注射1-萘基PP1(1-NA-PP1)(30 mg/kg)后,血浆和脑组织中均检测到该抑制剂,证实其可穿透血脑屏障[3] 2. 抑制PKCε介导的磷酸化:对AS-PKCε小鼠腹腔注射1-萘基PP1(1-NA-PP1)(25 mg/kg),可显著降低GABA_A γ2受体亚基在S327位点的磷酸化(Western blot检测)。均值±标准误结果显示,与溶媒组相比差异有统计学意义(每组n=5,p=0.0175)[3] 3. 减少乙醇消耗:1-萘基PP1(1-NA-PP1)(30 mg/kg,腹腔注射)可可逆性减少AS-PKCε小鼠的乙醇消耗。小鼠经溶媒注射习惯化后达到稳定饮酒基线,给药后乙醇消耗显著降低,给药48小时后抑制作用消失(每组n=18,p<0.05)[3] 4. 不影响乙醇偏好和饮水量:30 mg/kg 1-萘基PP1(1-NA-PP1)不改变AS-PKCε小鼠对乙醇的偏好(乙醇摄入/(乙醇摄入+水摄入)×100)和总饮水量(每组n=18)[3] 5. 调节味觉偏好:30 mg/kg 1-萘基PP1(1-NA-PP1)可降低AS-PKCε小鼠的糖精摄入量,但不影响奎宁摄入量(每组n=14,p<0.05)[3] 6. 不影响乙醇清除:30 mg/kg 1-萘基PP1(1-NA-PP1)不改变AS-PKCε小鼠的乙醇清除。腹腔注射1.5 g/kg乙醇后,不同时间点采集尾静脉血,酶法检测血乙醇浓度,结果显示与溶媒组无显著差异(每组n=7)[3] 7. 延长乙醇诱导的共济失调:30 mg/kg 1-萘基PP1(1-NA-PP1)(腹腔注射)可延长AS-PKCε小鼠乙醇(1.5 g/kg)诱导的共济失调恢复时间。小鼠在5 rpm旋转棒上的坠落时间(最长300秒)显著延长,恢复时间(可在棒上停留300秒)显著增加(溶媒组n=11,抑制剂组n=13,p=0.0014)[3] 8. 延长乙醇诱导的翻正反射丧失(LORR):30 mg/kg 1-萘基PP1(1-NA-PP1)(腹腔注射)可增加AS-PKCε小鼠乙醇(3.6 g/kg)诱导的LORR持续时间。LORR定义为小鼠失去自主翻正能力至恢复翻正能力(30秒内3次成功翻正)的时间,抑制剂组显著长于溶媒组(溶媒组n=25,抑制剂组n=26,p=0.0014)[3] 9. 对野生型小鼠无显著作用:20/30 mg/kg 1-萘基PP1(1-NA-PP1)(腹腔注射)不显著改变野生型C57BL/6NTac小鼠的乙醇消耗;仅在C57BL/6J小鼠中,20 mg/kg剂量轻微增加乙醇消耗,30 mg/kg剂量无影响(每组n=7–10)[3] 10. 不影响野生型小鼠的乙醇诱导行为:30 mg/kg 1-萘基PP1(1-NA-PP1)不改变野生型小鼠乙醇诱导的共济失调恢复时间或LORR持续时间(每组n=8)[3] |
| 酶活实验 |
体外放射学PKD1筛选试验[1]
采用体外放射激酶法筛选80个化合物库,在1µM浓度下检测PKD1抑制活性。用1.2µM的HDAC5肽作为底物进行反应。在含有50µL含有50 mM Tris-HCl、pH 7.5、4 mM MgCl2和10 mM β-巯基乙醇的激酶缓冲液中,用1µCi [γ-32P] ATP、25µM ATP、50 ng纯化的重组PKD1进行激酶反应,检测HDAC5的磷酸化。反应在30℃下孵育10分钟,取25µL的反应液滴在Whatman P81滤纸上。滤纸在0.5%磷酸中洗涤3次,风干后用Beckman LS6500多功能闪烁计数器计数。使用GraphPad Prism软件5.0绘制PKD1抑制百分比图。 体外放射PKC和CAMKIIα激酶测定[1] PKC激酶检测是通过将1µCi [γ-32P]ATP、20µM ATP、50 ng纯化PKCα或PKCδ和5µg髓鞘碱性蛋白4 - 14、0.25 mg/mL牛血清白蛋白、0.1 mg/mL磷脂酰胆碱/磷脂酰丝氨酸(80/20%)(1µM)、1µM二丁酸磷在50µL含有50 mM Tris-HCl、pH 7.5、4 mM MgCl2和10 mM β-巯基乙醇的激酶缓冲液中共孵育进行的。CAMK实验中,50 ng CAMKIIα和2µg syntide-2底物在50µL激酶缓冲液中与0.1 mM MgCl2、1µCi [γ-32P] ATP、70µM ATP孵育。0.5 mM CaCl2和30 ng/µL钙调素在冰上预孵育15 min后加入激酶反应。反应在30℃下孵育10 min,取25µL的反应液滴在Whatman P81滤纸上。滤纸在0.5%磷酸中洗涤3次,风干后用Beckman LS6500多功能闪烁计数器计数。 体外激酶测定[2] 我们在低ATP浓度(10 nM)的0.2µCiµl-1 [γ-32P]ATP存在下进行了体外激酶实验(Cdc28除外),因此IC50值代表了抑制常数(Ki)的粗略测量。抑制剂IC50值的测定方法如上所述。 纯化的Cdc28-His6 (1 nM)和MBP-Clb2 (3 nM)在25µl反应混合物中23°C孵育10分钟,反应混合物中含有5µg组蛋白H1, 1µCi的[γ-32P]ATP(1µCi / 10µM和1µCi / 1 mM),以及不同浓度的化合物9激酶缓冲液(25 mM hepe - naoh pH 7.4, 10 mM NaCl, 10 mM MgCl2和1 mM二硫代索糖醇)。反应产物用15% SDS-PAGE进行分析,然后进行放射自显影。为了测定Cdc28的动力学常数,不同浓度的[γ-32P]ATP(1µCi / 100µM)孵育和分析如上所述。 1. PKD亚型放射学激酶实验:制备重组人PKD1、PKD2或PKD3,构建含重组激酶、适宜底物、ATP(含放射性标记)和10种不同浓度1-萘基PP1(1-NA-PP1)的反应体系。在激酶活性最佳条件下孵育,放射检测器测量底物中放射性磷酸的掺入量以定量激酶活性。以抑制剂浓度为横坐标、活性为纵坐标绘制曲线,计算IC₅₀(至少3次独立实验,每个浓度3次重复,结果以均值±标准误表示)[2] 2. PKD1的ATP竞争性实验:构建含重组PKD1、底物和系列递增浓度ATP的反应体系,每个ATP浓度组加入不同浓度的1-萘基PP1(1-NA-PP1)。孵育完成后放射法检测PKD1活性,绘制双倒数图(1/活性 vs 1/ATP浓度),若图中线条平行,则证实抑制剂与ATP竞争结合激酶的ATP口袋[2] |
| 细胞实验 |
MTT试验[1]
PC3细胞接种于96孔板(3000个细胞/孔),贴壁过夜。然后将细胞在含有0.7-100µM抑制剂的培养基中孵育72 h,以2mg /mL浓度在PBS中制备3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化甲基噻唑基四唑(MTT)溶液,通过0.2µM过滤器过滤灭菌,并用铝箔包裹以遮光。每孔加入50µL MTT溶液,37℃孵育4 h。然后去除培养基,每孔加入200µL DMSO。振荡混合5 min,在570 nm处测定光密度。 细胞增殖试验和细胞周期分析[1] 通过台盼蓝染色计数活细胞数(如前所述)来测量PC3细胞的增殖。按照描述进行细胞周期分析。简单地说,用指定的化合物在30µM下处理PC3细胞72 h,然后在70%的冷冻乙醇中固定过夜,然后用碘化丙啶标记。标记的细胞使用FACSCalibur流式细胞仪 进行分析。 创面愈合试验[1] PC3或DU145细胞在6孔板中培养融合。迁移是通过用移液管尖端刮擦单层开始的,形成一个“伤口”。在培养基中加入指定浓度的化合物,并立即在10倍物镜的倒置相差显微镜下对伤口进行成像。24小时后,拍摄最终图像。测量创面间隙,计算创面愈合率。平均伤口愈合百分比是根据至少6次伤口间隙测量来确定的。 基质侵袭试验[1] 将DU145细胞(4.0×104 cells/ml)在含有0.1%胎牛血清(FBS)的RPMI中接种到BioCoat对照植入物(孔径8µm)或BioCoat Matrigel侵袭植入物(带有Matrigel涂层过滤器)的顶室中。为了刺激侵入,植入物下腔的介质中含有20%的FBS。上、下腔均加入浓度为30µM的抑制剂,细胞孵育22 h。孵育后,用棉签去除无创细胞,将有创细胞用100%甲醇固定,并用0.4%苏木精染色。染色后,在200倍放大镜下计数。侵染率由侵染基质的细胞数相对于通过对照插入的细胞数在5个区域内的细胞计数来确定。 1. LNCaP细胞PKD1激活的Western blot检测:LNCaP细胞接种于培养板,过夜贴壁后用不同剂量1-萘基PP1(1-NA-PP1)预处理45分钟,再用10 nM PMA刺激20分钟。适宜裂解液裂解细胞,提取总蛋白并定量。SDS-PAGE电泳后转膜,一抗孵育(抗p-S⁹¹⁶-PKD1、p-S⁷⁴⁴/⁷⁴⁸-PKD1和微管蛋白(内参)),二抗孵育后化学发光显影。光密度定量条带,绘制浓度-响应曲线计算IC₅₀[2] 2. PC3细胞增殖实验:24孔板中以3复孔接种PC3细胞(5×10³个细胞/孔),过夜贴壁。第1天用血细胞计数板计数(基线),加入1-萘基PP1(1-NA-PP1)(10 μM)或DMSO,每2天更换培养基和抑制剂,连续5天每日计数,计算每时间点3复孔均值,绘制细胞数量-时间曲线评估增殖[2] 3. MTT细胞活力实验:96孔板接种PC3细胞(3000个细胞/孔),过夜贴壁后加入0.3–100 μM 1-萘基PP1(1-NA-PP1)(DMSO为对照),孵育72小时。每孔加MTT溶液(5 mg/mL)孵育4小时,弃上清后加DMSO溶解甲臜,酶标仪测570 nm吸光度。计算相对细胞活力,IC₅₀为2次独立实验的均值[2] 4. 流式细胞术细胞周期分析:10 μM 1-萘基PP1(1-NA-PP1)或DMSO处理PC3细胞48小时,收集细胞用PBS洗涤,70%乙醇-20°C固定过夜。离心弃乙醇,用含碘化丙啶(50 μg/mL)和RNase A(100 μg/mL)的PBS重悬,37°C孵育30分钟。流式细胞术分析G1、S、G2/M期细胞比例,非配对t检验分析统计学差异[2] 5. 划痕愈合迁移实验:PC3细胞在6孔板中培养至100%汇合,无菌枪头制造均匀划痕,PBS洗涤后立即成像(0小时)。加入含30 μM 1-萘基PP1(1-NA-PP1)或DMSO的培养基,孵育22小时后再次成像。图像分析软件测量划痕面积,计算伤口愈合百分比[2] 6. Matrigel侵袭实验:Transwell小室用Matrigel包被并37°C孵育1小时成胶,上室接种DU145细胞(5×10⁴个细胞/小室)和含30 μM 1-萘基PP1(1-NA-PP1)或DMSO的培养基,下室加完全培养基。孵育20小时后,棉签去除上室非侵袭细胞,下室细胞用甲醇固定、苏木精染色,6个随机视野计数,计算相对侵袭率[2] 7. PKD过表达拯救实验:60 mm培养皿接种PC3细胞(5×10⁵个细胞),过夜孵育后用含PKD1、PKD3或空载体的腺病毒(50–100 MOI)感染。24小时后消化细胞,96孔板(活力实验)或Transwell小室(侵袭实验)接种,用10/30 μM 1-萘基PP1(1-NA-PP1)处理后进行MTT或侵袭实验,Western blot验证PKD过表达[2] 8. HEK293细胞突变PKD1磷酸化实验:转染HEK293细胞以表达Flag标记的野生型或突变型PKD1,转染2天后血清饥饿24小时,递增浓度1-萘基PP1(1-NA-PP1)预处理后PMA刺激,Western blot检测p-S⁹¹⁶-PKD1、Flag和微管蛋白[2] |
| 动物实验 |
Administration of 1-NA-PP1 [3]
For ethanol, saccharin, and quinine consumption studies, we dissolved 1-NA-PP1 in 100% DMSO at 20 or 30mg/ml and then diluted it 20-fold in deionized water containing 10% Tween-80 with sonication. For studies using oral administration, we prepared 1-NA-PP1 as a 100mM stock solution in 100% DMSO by gentle heating and sonication. This stock was diluted to 500µM in water containing 1% cremophor-RH40 and 2g/L sucralose to increase palatability. Control animals received an equivalent amount of DMSO vehicle in cremophor-sucralose-water. 1-NA-PP1 food pellets (1g/kg) were obtained from Research Diets (New Brunswick, NJ). Control food pellets contained an equivalent amount of vehicle (DMSO). To determine the effects of 1-NA-PP1 on protein phosphorylation, we dissolved 1-NA-PP1 in vehicle containing 5% DMSO and 20% Cremophor EL 1. Generation of AS-PKCε knock-in mice: Design a targeting vector to introduce the M486A point mutation (gatekeeper mutation) in exon 11 of the mouse Prkce gene, including a neomycin resistance (Neo) cassette (flanked by loxP sites) for positive selection and a diphtheria toxin A (DTA) cassette for negative selection. Transfect the vector into embryonic stem cells, select clones with homologous recombination, and excise the Neo cassette using Cre-recombinase. Generate chimeric mice, breed to obtain germline transmission, and confirm genotype via PCR of tail DNA. Verify PKCε expression levels and brain distribution via Western blot (hippocampus) and immunohistochemistry [3] 2. Pharmacokinetic assay for 1-Naphthyl PP1 (1-NA-PP1): Administer 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg) to AS-PKCε mice via intraperitoneal injection. At different time points post-injection (e.g., 0.25, 0.5, 1, 2, 4, 8, 12 hours), euthanize mice (n=3 per time point), collect blood (to prepare plasma) and whole brain. Extract 1-Naphthyl PP1 (1-NA-PP1) from plasma and brain tissues using appropriate solvents, and quantify concentrations via a validated analytical method (e.g., HPLC-MS/MS) to generate plasma and brain concentration-time profiles [3] 3. GABA_A γ2 phosphorylation assay in mice: Inject AS-PKCε mice with 1-Naphthyl PP1 (1-NA-PP1) (25 mg/kg, intraperitoneal) or vehicle (DMSO in saline). After 1 hour, euthanize mice (n=5 per group), dissect brain tissues (e.g., cortex, hippocampus), and prepare protein extracts. Perform Western blot with antibodies against phosphorylated GABA_A γ2 (p-S327) and total GABA_A γ2. Quantify band intensities via densitometry, calculate the ratio of p-S327 to total GABA_A γ2, and compare between inhibitor and vehicle groups using unpaired t-test [3] 4. Ethanol consumption assay in mice: House AS-PKCε mice individually with free access to water and 10% ethanol (v/v) for 2 weeks to establish stable drinking behavior. Habituate mice to intraperitoneal injections of vehicle (3 times/week) until ethanol consumption is stable. Administer 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg, intraperitoneal) or vehicle, and measure ethanol and water intake daily for 3 days (including 48 hours post-administration to assess reversibility). Calculate ethanol preference as (ethanol intake / (ethanol intake + water intake)) × 100. Use Dunnett’s test for statistical analysis (n=18 per group) [3] 5. Taste preference assays (saccharin and quinine): For saccharin preference, provide AS-PKCε mice with free access to water and 0.1% saccharin solution for 3 days to establish baseline intake. Administer 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg, intraperitoneal) or vehicle, and measure saccharin and water intake for 24 hours. For quinine preference, use 0.04% quinine solution and repeat the protocol. Calculate preference ratio as (taste solution intake / (taste solution intake + water intake)) × 100 (n=14 per group) [3] 6. Ethanol clearance assay: Inject AS-PKCε mice with 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg, intraperitoneal) or vehicle. Thirty minutes later, administer ethanol (1.5 g/kg, intraperitoneal). At 15, 30, 60, 90, and 120 minutes post-ethanol injection, collect blood samples from the tail vein. Measure blood ethanol concentration using an enzymatic assay kit. Calculate ethanol clearance rate as the slope of the concentration-time curve (n=7 per group) [3] 7. Ethanol-induced ataxia assay: Inject AS-PKCε or wild-type mice with 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg, intraperitoneal) or vehicle. Thirty minutes later, administer ethanol (1.5 g/kg, intraperitoneal). Place mice on a rotating rod (5 rpm) and measure the time until they fall off (maximum 300 seconds) at 15, 30, 45, 60, and 90 minutes post-ethanol injection. The recovery time is defined as the time when mice can remain on the rod for 300 seconds [3] 8. Ethanol-induced LORR assay: Inject AS-PKCε or wild-type mice with 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg, intraperitoneal) or vehicle. Thirty minutes later, administer ethanol (3.6 g/kg, intraperitoneal) and place mice in a supine position. The onset of LORR is the time until mice lose the ability to right themselves; the duration is the time from onset to recovery of righting reflex (mice can right themselves three times within 30 seconds) [3] |
| 药代性质 (ADME/PK) |
To determine the abundance and half-life of 1-NA-PP1 in plasma and brain, a pharmacokinetic study was performed following intraperitoneal administration of 30mg/kg 1-NA-PP1 in 5% DMSO and 10% Tween-80 to wild type C57BL/6J mice (Fig. 2A). Plasma levels of 1NA-PP1 reached 7.3 ± 0.43µM thirty minutes after injection and declined biphasically (R2= 0.94) with half-lives of 0.47 and 11.62 hours (Fig. 2A). Brain levels reached 2167 ± 85 ng/g (~6.8 ± 0.27µM) one hour after injection and declined in a single-phase (R2 = 0.93) with a half-life of 0.57 hours (Fig. 2B). These results indicate that 1-NA-PP1 enters the brain rapidly and efficiently after intraperitoneal administration and achieves concentrations predicted to inhibit AS-PKCε (Ki = 18.7nM) based on in vitro studies (Qi et al., 2007). [3]
Plasma and brain concentrations of 1-NA-PP1 were also determined following repeated oral administration. Wild type C57BL/6N mice were provided food pellets containing 1g/kg 1-NA-PP1 and water containing 500µM 1-NA-PP1 in 1% Cremophor-RH40 and 0.2% sucralose. Control animals were fed food and water containing the corresponding vehicles. Mice were sacrificed after 3 days and the concentration of 1-NA-PP1 was determined by LC-MS/MS. Oral administration of 1-NA-PP1 yielded a plasma concentration of 117 ± 23nM (n=5) and brain concentration of 140 ± 54ng/g protein (~ 441 ± 172nM; n=5). These results indicate that repeated administration of 1-NA-PP1 in food and water leads to levels of 1-NA-PP1 in the brain and plasma predicted to inhibit AS-PKCε (Qi et al., 2007). [3] To determine whether systemic administration of 1-NA-PP1 inhibits AS-PKCε-mediated phosphorylation in the brain, we examined phosphorylation of the GABAA receptor γ2 subunit since we previously found that PKCε phosphorylates this subunit at S327 (Qi et al., 2007). We administered 1-NA-PP1 by intraperitoneal injection rather than orally in this experiment to better control the dosage relative to the timing of tissue collection. AS-PKCε mice were administered 25mg/kg 1-NA-PP1 or vehicle and sacrificed 1 hour later. Although we used a different vehicle (5%DMSO/20% Cremophor-EL) to dissolve 1-NA-PP1 for this experiment, pharmacokinetic analyses after intraperitoneal injection of 30mg/kg 1-NA-PP1 in this vehicle revealed plasma (6.47 ± 0.25µM; n = 2) and brain concentrations (2055 ± 455ng/g; ~4.43 ± 2.03µM; n = 2) similar to those observed for 1-NA-PP1 dissolved in 5%DMSO/10% Tween-80. Compared with vehicle-injected mice, there was a 33% reduction in γ2-S(P)327 phosphoimmunoreactivity in the striatum of 1-NA-PP1-treated mice (Fig. 3). [3] 1. Blood-brain barrier penetration: Intraperitoneal administration of 1-Naphthyl PP1 (1-NA-PP1) (30 mg/kg) to AS-PKCε mice resulted in detectable concentrations in both plasma and brain. Plasma concentrations peaked at approximately 1 hour post-injection (mean ~800 ng/mL) and declined exponentially thereafter, with a half-life of ~2 hours. Brain concentrations peaked at ~1.5 hours post-injection (mean ~300 ng/mL) and remained above the threshold for PKCε inhibition for at least 4 hours, confirming central nervous system penetration [3] 2. Concentration distribution: At 0.5 hours post-injection, mean plasma concentration of 1-Naphthyl PP1 (1-NA-PP1) was ~600 ng/mL, and mean brain concentration was ~200 ng/mL; at 4 hours post-injection, plasma concentration was ~100 ng/mL, and brain concentration was ~50 ng/mL. The brain-to-plasma concentration ratio was approximately 0.3–0.4 across all time points, indicating consistent distribution into the brain [3] |
| 参考文献 |
|
| 其他信息 |
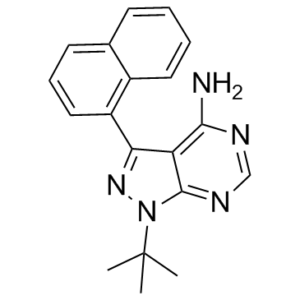
1-NA-PP1 is a pyrazolopyrimidine. It has a role as a tyrosine kinase inhibitor.
\n\nThe emergence of protein kinase D (PKD) as a potential therapeutic target for several diseases including cancer has triggered the search for potent, selective, and cell-permeable small molecule inhibitors. In this study, we describe the identification, in vitro characterization, structure-activity analysis, and biological evaluation of a novel PKD inhibitory scaffold exemplified by 1-naphthyl PP1 (1-NA-PP1). 1-NA-PP1 and IKK-16 were identified as pan-PKD inhibitors in a small-scale targeted kinase inhibitor library assay. Both screening hits inhibited PKD isoforms at about 100 nM and were ATP-competitive inhibitors. Analysis of several related kinases indicated that 1-NA-PP1 was highly selective for PKD as compared to IKK-16. SAR analysis showed that 1-NA-PP1 was considerably more potent and showed distinct substituent effects at the pyrazolopyrimidine core. 1-NA-PP1 was cell-active, and potently blocked prostate cancer cell proliferation by inducing G2/M arrest. It also potently blocked the migration and invasion of prostate cancer cells, demonstrating promising anticancer activities on multiple fronts. Overexpression of PKD1 or PKD3 almost completely reversed the growth arrest and the inhibition of tumor cell invasion caused by 1-NA-PP1, indicating that its anti-proliferative and anti-invasive activities were mediated through the inhibition of PKD. Interestingly, a 12-fold increase in sensitivity to 1-NA-PP1 could be achieved by engineering a gatekeeper mutation in the active site of PKD1, suggesting that 1-NA-PP1 could be paired with the analog-sensitive PKD1(M659G) for dissecting PKD-specific functions and signaling pathways in various biological systems.[1] \n\nProtein kinases have proved to be largely resistant to the design of highly specific inhibitors, even with the aid of combinatorial chemistry. The lack of these reagents has complicated efforts to assign specific signalling roles to individual kinases. Here we describe a chemical genetic strategy for sensitizing protein kinases to cell-permeable molecules that do not inhibit wild-type kinases. From two inhibitor scaffolds, we have identified potent and selective inhibitors for sensitized kinases from five distinct subfamilies. Tyrosine and serine/threonine kinases are equally amenable to this approach. We have analysed a budding yeast strain carrying an inhibitor-sensitive form of the cyclin-dependent kinase Cdc28 (CDK1) in place of the wild-type protein. Specific inhibition of Cdc28 in vivo caused a pre-mitotic cell-cycle arrest that is distinct from the G1 arrest typically observed in temperature-sensitive cdc28 mutants. The mutation that confers inhibitor-sensitivity is easily identifiable from primary sequence alignments. Thus, this approach can be used to systematically generate conditional alleles of protein kinases, allowing for rapid functional characterization of members of this important gene family.[2] \n\nReducing expression or inhibiting translocation of protein kinase C epsilon (PKCε) prolongs ethanol intoxication and decreases ethanol consumption in mice. However, we do not know if this phenotype is due to reduced PKCε kinase activity or to impairment of kinase-independent functions. In this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε catalytic activity reduces ethanol consumption. We generated ATP analog-specific PKCε (AS-PKCε) knock-in mice harboring a point mutation in the ATP binding site of PKCε that renders the mutant kinase highly sensitive to inhibition by 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine (1-NA-PP1). Systemically administered 1-NA-PP1 readily crossed the blood brain barrier and inhibited PKCε-mediated phosphorylation. 1-NA-PP1 reversibly reduced ethanol consumption by AS-PKCε mice but not by wild type mice lacking the AS-PKCε mutation. These results support the development of inhibitors of PKCε catalytic activity as a strategy to reduce ethanol consumption, and they demonstrate that the AS- PKCε mouse is a useful tool to study the role of PKCε in behavior.[3] 1. 1-Naphthyl PP1 (1-NA-PP1) belongs to the pyrazolopyrimidine chemical scaffold and was originally developed as an inhibitor for analog-sensitive mutant Src kinases; it was later identified as a potent pan-PKD inhibitor through a targeted kinase inhibitor library screen [2] 2. Structure-activity relationship (SAR) analysis of 1-Naphthyl PP1 (1-NA-PP1) showed that the naphthyl group at the pyrazolopyrimidine core is critical for PKD inhibition, and substitutions at this position significantly affect potency and selectivity [2] 3. The gatekeeper mutation (M659G) in PKD1 enlarges the ATP-binding pocket, allowing accommodation of the bulky naphthyl moiety of 1-Naphthyl PP1 (1-NA-PP1)—this structural modification is the basis for the 12-fold increase in sensitivity, enabling specific dissection of PKD signaling pathways [2] 4. 1-Naphthyl PP1 (1-NA-PP1) is cell-permeable, which is essential for its activity in intact cells (e.g., LNCaP, PC3, HEK293) and in vivo (crossing the blood-brain barrier) [2, 3] 5. The chemical name of 1-Naphthyl PP1 (1-NA-PP1) is 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine, with a PubChem CID of 4877 [3] 6. 1-Naphthyl PP1 (1-NA-PP1) specifically inhibits the catalytic activity of target kinases (PKD, AS-PKCε) without affecting their total protein expression levels, as confirmed by Western blot analysis of PKD1, PKD3, PKCε, and GABA_A γ2 in cells and mouse tissues [2, 3] |
| 分子式 |
C19H19N5
|
|---|---|
| 分子量 |
317.3877
|
| 精确质量 |
317.164
|
| CAS号 |
221243-82-9
|
| 相关CAS号 |
1-Naphthyl PP1 hydrochloride;956025-47-1
|
| PubChem CID |
4877
|
| 外观&性状 |
Light yellow to khaki solid
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
527.8±45.0 °C at 760 mmHg
|
| 熔点 |
219-222ºC
|
| 闪点 |
273.0±28.7 °C
|
| 蒸汽压 |
0.0±1.4 mmHg at 25°C
|
| 折射率 |
1.688
|
| LogP |
3.88
|
| tPSA |
69.62
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
448
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N1(C2C(=C(N([H])[H])N=C([H])N=2)C(C2=C([H])C([H])=C([H])C3=C([H])C([H])=C([H])C([H])=C23)=N1)C(C([H])([H])[H])(C([H])([H])[H])C([H])([H])[H]
|
| InChi Key |
XSHQBIXMLULFEV-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H19N5/c1-19(2,3)24-18-15(17(20)21-11-22-18)16(23-24)14-10-6-8-12-7-4-5-9-13(12)14/h4-11H,1-3H3,(H2,20,21,22)
|
| 化学名 |
1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine
|
| 别名 |
1-Naphthyl PP1; 221243-82-9; 1-NAPHTHYL PP1; 1-NA-PP1; 1-(tert-Butyl)-3-(naphthalen-1-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; 4-Amino-1-tert-butyl-3-(1'-naphthyl)pyrazolo[3,4-d]pyrimidine; 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine; 1-(1,1-dimethylethyl)-3-(1-naphthalenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; C19H19N5; 1-NA-PP 1
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: 9~12.5 mg/mL (28.4~39.4 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 1.25 mg/mL (3.94 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 12.5 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 1.25 mg/mL (3.94 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 12.5 mg/mL 澄清 DMSO 储备液加入到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.1507 mL | 15.7535 mL | 31.5070 mL | |
| 5 mM | 0.6301 mL | 3.1507 mL | 6.3014 mL | |
| 10 mM | 0.3151 mL | 1.5753 mL | 3.1507 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。