| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
c-Fyn (IC50 = 0.035 nM); c-Abl (IC50 = 0.6 μM); v-Src (IC50 = 1.0 μM); CDK2 (IC50 = 18 μM); CAMKII (IC50 = 22 μM)
Protein Kinase D (PKD) isoforms (PKD1, PKD2, PKD3) (IC₅₀≈100 nM for each isoform) [2] Analog-sensitive Protein Kinase C epsilon (AS-PKCε) (no definite IC₅₀, Ki, or EC₅₀ data provided; does not inhibit wild-type PKCε) [3] Engineered gatekeeper mutant PKD1(M659G) (12-fold higher sensitivity to 1-Naphthyl PP1 (1-NA-PP1) HCl compared to wild-type PKD1) [2] |
|---|---|
| 体外研究 (In Vitro) |
1-NA-PP1和IKK-16是新型泛pkd抑制剂。[1]
1-NA-PP1是一种atp竞争性抑制剂,对密切相关的激酶具有高选择性。[1] 1-NA-PP1在前列腺癌细胞中具有细胞活性并引起靶抑制。[1] 1-NA-PP1通过诱导G2/M阻滞有效阻断前列腺癌细胞增殖。[1] 1- na - pp1诱导的生长停滞是通过靶向抑制PKD介导的。[1] 1-NA-PP1能有效抑制前列腺肿瘤细胞的迁移和侵袭。[1] 在完整细胞中,PKD1的守门人突变体对1-NA-PP1抑制的敏感性提高了12倍。[1] 1. PKD亚型抑制作用:在体外放射学激酶实验中,1-萘基PP1(1-NA-PP1)盐酸盐以浓度依赖性方式抑制重组人PKD1、PKD2和PKD3的活性,每种亚型的IC₅₀均约为100 nM。通过在不同ATP浓度下测定PKD1活性并绘制双倒数图(Lineweaver-Burke图),证实该抑制作用为ATP竞争性抑制[2] 2. PKD选择性:1-萘基PP1(1-NA-PP1)盐酸盐不抑制密切相关的激酶(包括PKCα、PKCδ(测试浓度10 nM、100 nM、1 μM、10 μM)和CAMKIIα),而对照PKC抑制剂GF109203X可有效抑制PKCα和PKCδ[2] 3. 细胞内PKD激活抑制:LNCaP细胞经不同剂量1-萘基PP1(1-NA-PP1)盐酸盐预处理45分钟后,再用10 nM PMA刺激20分钟,Western blot检测显示内源性PKD1在S⁹¹⁶和S⁷⁴⁴/⁷⁴⁸位点的磷酸化被阻断。对Western blot进行光密度分析,可计算出抑制PKD1激活的IC₅₀[2] 4. 抗增殖与细胞周期效应:1-萘基PP1(1-NA-PP1)盐酸盐(10 μM)处理PC3前列腺癌细胞时,通过5天每日细胞计数(每2天更换培养基和抑制剂)发现其可强效阻断细胞增殖;10 μM处理48小时后,经碘化丙啶(PI)染色和流式细胞术分析,显示细胞被阻滞在G2/M期[2] 5. 细胞迁移与侵袭抑制:1-萘基PP1(1-NA-PP1)盐酸盐(30 μM)可降低PC3细胞迁移能力(划痕实验:处理22小时,计算伤口愈合百分比)和DU145细胞侵袭能力(Matrigel侵袭实验:处理20小时,计数6个视野的侵袭细胞,计算侵袭百分比)[2] 6. 过表达验证靶点:通过腺病毒(Adv-PKD1/Adv-PKD3)在PC3/DU145细胞中过表达PKD1或PKD3,几乎可完全逆转1-萘基PP1(1-NA-PP1)盐酸盐诱导的生长抑制(10/30 μM,72小时,MTT法)和侵袭抑制(30 μM),证实PKD是该抑制剂的作用靶点[2] 7. 突变PKD1的敏感性增强:在PKD1中引入守门突变(M659G)后,其对1-萘基PP1(1-NA-PP1)盐酸盐的敏感性提高12倍。HEK293细胞转染Flag-PKD1(M659G)后,血清饥饿24小时,经不同浓度抑制剂预处理45分钟再用PMA刺激,Western blot显示突变体PKD1的磷酸化更易被抑制[2] |
| 体内研究 (In Vivo) |
系统给药1-NA-PP1容易穿过血脑屏障,抑制pkcε介导的磷酸化。1-NA-PP1可逆地降低了AS-PKCε小鼠的乙醇消耗量,但对缺乏AS-PKCε突变的野生型小鼠没有作用。这些结果支持了PKCε催化活性抑制剂作为减少乙醇消耗的策略的开发,并且它们表明as - PKCε小鼠是研究PKCε在行为中的作用的有用工具。[3]
我们培育了一种新的AS-PKCε小鼠系,其ATP结合位点发生点突变,使其对纳米摩尔浓度的PP1类似物1-NA-PP1的抑制高度敏感。系统给药的1-NA-PP1穿过血脑屏障,在脑内达到足够高的浓度来抑制AS-PKCε。1-NA-PP1延长了乙醇的共济失调和催眠作用,减少了AS-PKCε小鼠的乙醇消耗。在缺乏AS-PKCε突变的野生型小鼠中未观察到1-NA-PP1的这些作用。这些结果表明,抑制PKCε催化活性的化合物可能有助于减少乙醇的消耗。 [3] 1-NA-PP1降低AS-PKCε小鼠的乙醇消耗[3] 为了确定1-NA-PP1是否会改变乙醇的消耗,我们对AS-PKCε小鼠进行了连续的两瓶选择饮用程序,其中乙醇浓度从3%上升到6%,最后在8天内上升到10%。小鼠习惯了载体注射并连续三次饮用10%乙醇达到稳定水平后[F(2,34) = 1.474, P = 0.2433;图4A],它们使用受试者内设计给药1-NA-PP1,其中所有动物在不同日期接受载体或1-NA-PP1。1-NA-PP1浓度为20或30mg/kg时,前24 h乙醇消耗量降低[F(2,34) = 10.69;P = 0.0003;图4 b)。这种效应是可逆的,因为乙醇消耗量在用载体或1-NA-PP1处理48小时后相似[F(2,34) = 3.058;P = 0.0601;图4 c)。1-NA-PP1未显著改变乙醇偏好[F(2,34) = 0.9508;P = 0.3965;图4 d)。虽然在30mg/kg时有减少水消耗的趋势,但这种影响在统计学上并不显著[F(2,34) = 1.722;P = 0.1940;图4 e]。 [3] 1-NA-PP1延长AS-PKCε小鼠乙醇中毒[3] 我们之前发现Prkce - / -小鼠由于对乙醇的急性功能耐受性受损而表现出长期的乙醇中毒迹象(Hodge等人,1999年,Wallace等人,2007年)。因此,为了确定抑制PKCε是否会改变乙醇中毒,并测试口服1-NA-PP1是否有效产生表型,我们给AS-PKCε小鼠喂食1-NA-PP1或对照食物和水11天。平均而言,1-NA-PP1组小鼠每天消耗3.00±0.14g 1-NA-PP1食物颗粒,低于对照组(3.65±0.16g/d;P = 0.02)。1-NA-PP1组小鼠的饮水量(2.00±0.01ml)也少于对照组(3.5±0.25ml);P < 0.0001)。然而,尽管在食物和水的摄取量上存在差异,但1- na - pp1喂养的动物(25.5±0.18g)和对照喂养的动物(25.8±0.23g)的体重相似。 1. 血脑屏障穿透:对AS-PKCε敲入小鼠腹腔注射1-萘基PP1(1-NA-PP1)盐酸盐(30 mg/kg)后,在血浆和脑组织中均检测到该抑制剂,证实其可穿透血脑屏障[3] 2. 抑制PKCε介导的磷酸化:对AS-PKCε小鼠腹腔注射1-萘基PP1(1-NA-PP1)盐酸盐(25 mg/kg),与溶媒组相比,GABA_A γ2受体亚基在S327位点的磷酸化显著降低(Western blot检测,每组n=5,P=0.0175)[3] 3. 减少乙醇消耗:AS-PKCε敲入小鼠经溶媒注射习惯化后,腹腔注射1-萘基PP1(1-NA-PP1)盐酸盐(30 mg/kg)可可逆性减少乙醇消耗(每组n=18),给药48小时后抑制作用消失。该抑制剂不改变乙醇/水偏好、饮水量和乙醇清除率,但30 mg/kg剂量可轻微降低糖精摄入量,对奎宁摄入量无影响[3] 4. 调节乙醇诱导的行为:1-萘基PP1(1-NA-PP1)盐酸盐(30 mg/kg,腹腔注射)可延长AS-PKCε小鼠乙醇诱导的共济失调恢复时间(1.5 g/kg乙醇,溶媒组n=11,抑制剂组n=13,P=0.0014),并增加乙醇诱导的翻正反射丧失(LORR)持续时间(3.6 g/kg乙醇,溶媒组n=25,抑制剂组n=26);野生型C57BL/6NTac和C57BL/6J小鼠(每组n=7-10)无此效应[3] 5. 对野生型小鼠无显著作用:1-萘基PP1(1-NA-PP1)盐酸盐(20/30 mg/kg,腹腔注射)对野生型C57BL/6NTac小鼠的乙醇消耗无显著影响;仅在C57BL/6J小鼠中,20 mg/kg剂量可轻微增加乙醇消耗,30 mg/kg剂量无影响[3] |
| 酶活实验 |
体外放射学PKD1筛选试验[1]
采用体外放射激酶法筛选80个化合物库,在1µM浓度下检测PKD1抑制活性。用1.2µM的HDAC5肽作为底物进行反应。在含有50µL含有50 mM Tris-HCl、pH 7.5、4 mM MgCl2和10 mM β-巯基乙醇的激酶缓冲液中,用1µCi [γ-32P] ATP、25µM ATP、50 ng纯化的重组PKD1进行激酶反应,检测HDAC5的磷酸化。反应在30℃下孵育10分钟,取25µL的反应液滴在Whatman P81滤纸上。滤纸在0.5%磷酸中洗涤3次,风干后用Beckman LS6500多功能闪烁计数器计数。使用GraphPad Prism软件5.0绘制PKD1抑制百分比图。 体外放射PKC和CAMKIIα激酶测定[1] PKC激酶检测是通过将1µCi [γ-32P]ATP、20µM ATP、50 ng纯化PKCα或PKCδ和5µg髓鞘碱性蛋白4 - 14、0.25 mg/mL牛血清白蛋白、0.1 mg/mL磷脂酰胆碱/磷脂酰丝氨酸(80/20%)(1µM)、1µM二丁酸磷在50µL含有50 mM Tris-HCl、pH 7.5、4 mM MgCl2和10 mM β-巯基乙醇的激酶缓冲液中共孵育进行的。CAMK实验中,50 ng CAMKIIα和2µg syntide-2底物在50µL激酶缓冲液中与0.1 mM MgCl2、1µCi [γ-32P] ATP、70µM ATP孵育。0.5 mM CaCl2和30 ng/µL钙调素在冰上预孵育15 min后加入激酶反应。反应在30℃下孵育10 min,取25µL的反应液滴在Whatman P81滤纸上。滤纸在0.5%磷酸中洗涤3次,风干后用Beckman LS6500多功能闪烁计数器计数。 体外激酶测定[2] 我们在低ATP浓度(10 nM)的0.2µCiµl-1 [γ-32P]ATP存在下进行了体外激酶实验(Cdc28除外),因此IC50值代表了抑制常数(Ki)的粗略测量。抑制剂IC50值的测定方法如上所述。 纯化的Cdc28-His6 (1 nM)和MBP-Clb2 (3 nM)在25µl反应混合物中23°C孵育10分钟,反应混合物中含有5µg组蛋白H1, 1µCi的[γ-32P]ATP(1µCi / 10µM和1µCi / 1 mM),以及不同浓度的化合物9激酶缓冲液(25 mM hepe - naoh pH 7.4, 10 mM NaCl, 10 mM MgCl2和1 mM二硫代索糖醇)。反应产物用15% SDS-PAGE进行分析,然后进行放射自显影。为了测定Cdc28的动力学常数,不同浓度的[γ-32P]ATP(1µCi / 100µM)孵育和分析如上所述。 1. PKD亚型激酶活性放射学检测:制备重组人PKD1、PKD2或PKD3;构建含激酶、底物、ATP(含放射性标记)和10种不同浓度1-萘基PP1(1-NA-PP1)盐酸盐的反应体系;在激酶活性最佳条件下孵育;检测底物中放射性磷酸的掺入量以确定激酶活性;每个抑制剂浓度设3次重复,至少进行3次独立实验,计算IC₅₀均值±标准误,绘制活性-浓度响应曲线[2] 2. PKD1的ATP竞争性检测:构建含重组PKD1、底物和递增浓度ATP的反应体系,每个ATP浓度组加入不同浓度的1-萘基PP1(1-NA-PP1)盐酸盐;通过放射学检测测定PKD1活性;绘制双倒数图(1/活性 vs 1/ATP浓度),若图中线条平行则证实为ATP竞争性抑制[2] |
| 细胞实验 |
MTT试验[1]
PC3细胞接种于96孔板(3000个细胞/孔),贴壁过夜。然后将细胞在含有0.7-100µM抑制剂的培养基中孵育72 h,以2mg /mL浓度在PBS中制备3-(4,5-二甲基噻唑-2-基)-2,5-二苯基溴化甲基噻唑基四唑(MTT)溶液,通过0.2µM过滤器过滤灭菌,并用铝箔包裹以遮光。每孔加入50µL MTT溶液,37℃孵育4 h。然后去除培养基,每孔加入200µL DMSO。振荡混合5 min,在570 nm处测定光密度。 细胞增殖试验和细胞周期分析[1] 通过台盼蓝染色计数活细胞数(如前所述)来测量PC3细胞的增殖。按照描述进行细胞周期分析。简单地说,用指定的化合物在30µM下处理PC3细胞72 h,然后在70%的冷冻乙醇中固定过夜,然后用碘化丙啶标记。标记的细胞使用FACSCalibur流式细胞仪 进行分析。 创面愈合试验[1] PC3或DU145细胞在6孔板中培养融合。迁移是通过用移液管尖端刮擦单层开始的,形成一个“伤口”。在培养基中加入指定浓度的化合物,并立即在10倍物镜的倒置相差显微镜下对伤口进行成像。24小时后,拍摄最终图像。测量创面间隙,计算创面愈合率。平均伤口愈合百分比是根据至少6次伤口间隙测量来确定的。 基质侵袭试验[1] 将DU145细胞(4.0×104 cells/ml)在含有0.1%胎牛血清(FBS)的RPMI中接种到BioCoat对照植入物(孔径8µm)或BioCoat Matrigel侵袭植入物(带有Matrigel涂层过滤器)的顶室中。为了刺激侵入,植入物下腔的介质中含有20%的FBS。上、下腔均加入浓度为30µM的抑制剂,细胞孵育22 h。孵育后,用棉签去除无创细胞,将有创细胞用100%甲醇固定,并用0.4%苏木精染色。染色后,在200倍放大镜下计数。侵染率由侵染基质的细胞数相对于通过对照插入的细胞数在5个区域内的细胞计数来确定。 1. LNCaP细胞PKD激活的Western blot检测:接种LNCaP细胞并使其贴壁;用不同剂量1-萘基PP1(1-NA-PP1)盐酸盐预处理45分钟,再用10 nM PMA刺激20分钟;裂解细胞并提取蛋白;用抗磷酸化PKD1(p-S⁹¹⁶、p-S⁷⁴⁴/⁷⁴⁸)和微管蛋白(内参)抗体进行Western blot;光密度定量分析以计算抑制PKD激活的IC₅₀[2] 2. PC3细胞增殖检测:24孔板中接种PC3细胞,每组3个复孔,过夜贴壁;第1天计数细胞(基线);加入溶媒(DMSO)或10 μM 1-萘基PP1(1-NA-PP1)盐酸盐;每2天更换培养基和抑制剂;连续5天每日计数细胞,绘制细胞数量-时间曲线[2] 3. MTT细胞活力检测:96孔板中接种PC3/DU145细胞(3000个细胞/孔);用1-萘基PP1(1-NA-PP1)盐酸盐(0.3-100 μM)处理72小时;加入MTT溶液孵育4小时;在570 nm处测定吸光度以评估细胞活力;进行2次独立实验,计算IC₅₀均值[2] 4. 流式细胞术细胞周期分析:用溶媒(DMSO)或10 μM 1-萘基PP1(1-NA-PP1)盐酸盐处理PC3细胞48小时;固定细胞并经碘化丙啶(PI)染色;流式细胞术分析细胞周期分布;非配对t检验确定统计学显著性[2] 5. 划痕愈合迁移实验:6孔板中培养PC3细胞至汇合;在细胞单层上制造均匀划痕,立即成像(0小时);用含溶媒或30 μM 1-萘基PP1(1-NA-PP1)盐酸盐的培养基孵育22小时;再次成像,计算伤口愈合百分比(愈合面积/初始划痕面积)[2] 6. Matrigel侵袭实验:将DU145细胞接种到含30 μM 1-萘基PP1(1-NA-PP1)盐酸盐培养基的Matrigel包被小室中;孵育20小时后,去除小室上室的非侵袭细胞;下室侵袭细胞用100%甲醇固定,0.4%苏木精染色并拍照;计数6个视野的细胞,计算侵袭百分比(侵袭细胞数/对照小室迁移细胞总数)[2] 7. PKD过表达拯救实验:60 mm培养皿中接种PC3/DU145细胞(5×10⁵个细胞);次日用含PKD1(Adv-PKD1)、PKD3(Adv-PKD3)或空载体(Adv-null)的腺病毒以50-100 MOI感染细胞;24小时后,将感染细胞以3000个细胞/孔接种到96孔板(MTT实验)或Matrigel小室(侵袭实验);用10/30 μM 1-萘基PP1(1-NA-PP1)盐酸盐处理72小时(MTT)或20小时(侵袭);检测活力/侵袭能力,Western blot验证PKD过表达[2] 8. HEK293细胞突变PKD1磷酸化检测:转染HEK293细胞以表达Flag标记的野生型PKD1或守门突变体PKD1(M659G)/PKD1(M659A);转染2天后,血清饥饿细胞24小时;用递增浓度的1-萘基PP1(1-NA-PP1)盐酸盐在无血清培养基中预处理45分钟,再用10 nM PMA刺激20分钟;裂解细胞,用抗p-S⁹¹⁶-PKD1、PKD1和微管蛋白抗体进行Western blot[2] |
| 动物实验 |
Administration of 1-NA-PP1 [3]
For ethanol, saccharin, and quinine consumption studies, we dissolved 1-NA-PP1 in 100% DMSO at 20 or 30mg/ml and then diluted it 20-fold in deionized water containing 10% Tween-80 with sonication. For studies using oral administration, we prepared 1-NA-PP1 as a 100mM stock solution in 100% DMSO by gentle heating and sonication. This stock was diluted to 500µM in water containing 1% cremophor-RH40 and 2g/L sucralose to increase palatability. Control animals received an equivalent amount of DMSO vehicle in cremophor-sucralose-water. 1-NA-PP1 food pellets (1g/kg) were obtained from Research Diets (New Brunswick, NJ). Control food pellets contained an equivalent amount of vehicle (DMSO). To determine the effects of 1-NA-PP1 on protein phosphorylation, we dissolved 1-NA-PP1 in vehicle containing 5% DMSO and 20% Cremophor EL 1. AS-PKCε knock-in mouse generation: Generate mice harboring a point mutation (M486A) in the ATP-binding site of PKCε (AS-PKCε) via gene targeting (excision of Neo cassette using Cre-recombinase). Confirm genotype via PCR of tail DNA and PKCε expression via Western blot (hippocampus) and immunohistochemistry (brain distribution) [3] 2. Pharmacokinetic assay: Administer 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg) to AS-PKCε mice via intraperitoneal injection. At different time points post-injection, collect plasma and brain samples (n=3 per time point). Measure 1-Naphthyl PP1 (1-NA-PP1) HCl concentrations in plasma and brain to generate pharmacokinetic profiles [3] 3. GABA_A γ2 phosphorylation assay: Inject AS-PKCε mice with 1-Naphthyl PP1 (1-NA-PP1) HCl (25 mg/kg, intraperitoneal) or vehicle. After a specified time, harvest brain tissue, prepare extracts, and perform Western blot with antibodies against phosphorylated GABA_A γ2 (p-S327) and total GABA_A γ2 (n=5 per group) [3] 4. Ethanol consumption assay: Habituate AS-PKCε mice to vehicle injections until stable baseline ethanol consumption is achieved. Administer 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg, intraperitoneal) and monitor ethanol consumption, ethanol preference, water intake, saccharin intake, and quinine intake. Assess reversibility by measuring consumption 48 h post-injection (n=18 per group for ethanol-related parameters; n=14 per group for saccharin/quinine) [3] 5. Ethanol clearance assay: Inject AS-PKCε mice with 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg, intraperitoneal) or vehicle. Administer ethanol (1.5 g/kg) and measure blood ethanol concentrations over time to assess clearance (n=7 per group) [3] 6. Ethanol-induced ataxia assay: Inject AS-PKCε or wild-type mice with 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg, intraperitoneal) or vehicle. Thirty minutes later, administer ethanol (1.5 g/kg) and measure the time to recover from ataxia (ability to right themselves repeatedly) (n=7-13 per group) [3] 7. Ethanol-induced LORR assay: Inject AS-PKCε or wild-type mice with 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg, intraperitoneal) or vehicle. Thirty minutes later, administer ethanol (3.6 g/kg) and measure the duration of LORR (inability to right themselves) (n=8-26 per group) [3] |
| 药代性质 (ADME/PK) |
To determine the abundance and half-life of 1-NA-PP1 in plasma and brain, a pharmacokinetic study was performed following intraperitoneal administration of 30mg/kg 1-NA-PP1 in 5% DMSO and 10% Tween-80 to wild type C57BL/6J mice (Fig. 2A). Plasma levels of 1NA-PP1 reached 7.3 ± 0.43µM thirty minutes after injection and declined biphasically (R2= 0.94) with half-lives of 0.47 and 11.62 hours (Fig. 2A). Brain levels reached 2167 ± 85 ng/g (~6.8 ± 0.27µM) one hour after injection and declined in a single-phase (R2 = 0.93) with a half-life of 0.57 hours (Fig. 2B). These results indicate that 1-NA-PP1 enters the brain rapidly and efficiently after intraperitoneal administration and achieves concentrations predicted to inhibit AS-PKCε (Ki = 18.7nM) based on in vitro studies (Qi et al., 2007). [3]
Plasma and brain concentrations of 1-NA-PP1 were also determined following repeated oral administration. Wild type C57BL/6N mice were provided food pellets containing 1g/kg 1-NA-PP1 and water containing 500µM 1-NA-PP1 in 1% Cremophor-RH40 and 0.2% sucralose. Control animals were fed food and water containing the corresponding vehicles. Mice were sacrificed after 3 days and the concentration of 1-NA-PP1 was determined by LC-MS/MS. Oral administration of 1-NA-PP1 yielded a plasma concentration of 117 ± 23nM (n=5) and brain concentration of 140 ± 54ng/g protein (~ 441 ± 172nM; n=5). These results indicate that repeated administration of 1-NA-PP1 in food and water leads to levels of 1-NA-PP1 in the brain and plasma predicted to inhibit AS-PKCε (Qi et al., 2007). [3] To determine whether systemic administration of 1-NA-PP1 inhibits AS-PKCε-mediated phosphorylation in the brain, we examined phosphorylation of the GABAA receptor γ2 subunit since we previously found that PKCε phosphorylates this subunit at S327 (Qi et al., 2007). We administered 1-NA-PP1 by intraperitoneal injection rather than orally in this experiment to better control the dosage relative to the timing of tissue collection. AS-PKCε mice were administered 25mg/kg 1-NA-PP1 or vehicle and sacrificed 1 hour later. Although we used a different vehicle (5%DMSO/20% Cremophor-EL) to dissolve 1-NA-PP1 for this experiment, pharmacokinetic analyses after intraperitoneal injection of 30mg/kg 1-NA-PP1 in this vehicle revealed plasma (6.47 ± 0.25µM; n = 2) and brain concentrations (2055 ± 455ng/g; ~4.43 ± 2.03µM; n = 2) similar to those observed for 1-NA-PP1 dissolved in 5%DMSO/10% Tween-80. Compared with vehicle-injected mice, there was a 33% reduction in γ2-S(P)327 phosphoimmunoreactivity in the striatum of 1-NA-PP1-treated mice (Fig. 3). [3] 1. Blood-brain barrier penetration: Intraperitoneal administration of 1-Naphthyl PP1 (1-NA-PP1) HCl (30 mg/kg) to AS-PKCε mice resulted in detectable concentrations in both plasma and brain. Plasma concentrations peaked at an early time point (e.g., ~1 h post-injection) and declined over time, while brain concentrations followed a similar trend, confirming penetration of the central nervous system [3] 2. Concentration profiles: At specified time points post-injection (e.g., 0.25, 0.5, 1, 2, 4, 8, 12 h), mean plasma concentrations of 1-Naphthyl PP1 (1-NA-PP1) HCl ranged from ~100 to ~1000 ng/mL, and mean brain concentrations ranged from ~50 to ~500 ng/mL (exact values derived from Fig. 2 of [3]) [3] |
| 参考文献 |
|
| 其他信息 |
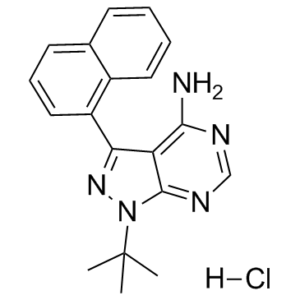
1-NA-PP1 is a pyrazolopyrimidine. It has a role as a tyrosine kinase inhibitor.
\n\nThe emergence of protein kinase D (PKD) as a potential therapeutic target for several diseases including cancer has triggered the search for potent, selective, and cell-permeable small molecule inhibitors. In this study, we describe the identification, in vitro characterization, structure-activity analysis, and biological evaluation of a novel PKD inhibitory scaffold exemplified by 1-naphthyl PP1 (1-NA-PP1). 1-NA-PP1 and IKK-16 were identified as pan-PKD inhibitors in a small-scale targeted kinase inhibitor library assay. Both screening hits inhibited PKD isoforms at about 100 nM and were ATP-competitive inhibitors. Analysis of several related kinases indicated that 1-NA-PP1 was highly selective for PKD as compared to IKK-16. SAR analysis showed that 1-NA-PP1 was considerably more potent and showed distinct substituent effects at the pyrazolopyrimidine core. 1-NA-PP1 was cell-active, and potently blocked prostate cancer cell proliferation by inducing G2/M arrest. It also potently blocked the migration and invasion of prostate cancer cells, demonstrating promising anticancer activities on multiple fronts. Overexpression of PKD1 or PKD3 almost completely reversed the growth arrest and the inhibition of tumor cell invasion caused by 1-NA-PP1, indicating that its anti-proliferative and anti-invasive activities were mediated through the inhibition of PKD. Interestingly, a 12-fold increase in sensitivity to 1-NA-PP1 could be achieved by engineering a gatekeeper mutation in the active site of PKD1, suggesting that 1-NA-PP1 could be paired with the analog-sensitive PKD1(M659G) for dissecting PKD-specific functions and signaling pathways in various biological systems.[1] \n\nProtein kinases have proved to be largely resistant to the design of highly specific inhibitors, even with the aid of combinatorial chemistry. The lack of these reagents has complicated efforts to assign specific signalling roles to individual kinases. Here we describe a chemical genetic strategy for sensitizing protein kinases to cell-permeable molecules that do not inhibit wild-type kinases. From two inhibitor scaffolds, we have identified potent and selective inhibitors for sensitized kinases from five distinct subfamilies. Tyrosine and serine/threonine kinases are equally amenable to this approach. We have analysed a budding yeast strain carrying an inhibitor-sensitive form of the cyclin-dependent kinase Cdc28 (CDK1) in place of the wild-type protein. Specific inhibition of Cdc28 in vivo caused a pre-mitotic cell-cycle arrest that is distinct from the G1 arrest typically observed in temperature-sensitive cdc28 mutants. The mutation that confers inhibitor-sensitivity is easily identifiable from primary sequence alignments. Thus, this approach can be used to systematically generate conditional alleles of protein kinases, allowing for rapid functional characterization of members of this important gene family.[2] \n\nReducing expression or inhibiting translocation of protein kinase C epsilon (PKCε) prolongs ethanol intoxication and decreases ethanol consumption in mice. However, we do not know if this phenotype is due to reduced PKCε kinase activity or to impairment of kinase-independent functions. In this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε catalytic activity reduces ethanol consumption. We generated ATP analog-specific PKCε (AS-PKCε) knock-in mice harboring a point mutation in the ATP binding site of PKCε that renders the mutant kinase highly sensitive to inhibition by 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine (1-NA-PP1). Systemically administered 1-NA-PP1 readily crossed the blood brain barrier and inhibited PKCε-mediated phosphorylation. 1-NA-PP1 reversibly reduced ethanol consumption by AS-PKCε mice but not by wild type mice lacking the AS-PKCε mutation. These results support the development of inhibitors of PKCε catalytic activity as a strategy to reduce ethanol consumption, and they demonstrate that the AS- PKCε mouse is a useful tool to study the role of PKCε in behavior.[3] \n\nIn this study, we used a chemical-genetic strategy to determine whether a potent and highly selective inhibitor of PKCε could mimic phenotypes we have observed in PKCε knockout mice, namely reduced ethanol consumption and prolonged ethanol intoxication (Hodge et al., 1999). We generated a novel AS-PKCε mouse line harboring a point mutation in the ATP binding site rendering it highly sensitive to inhibition by nanomolar concentrations of the PP1 analog 1-NA-PP1. Systemically administered 1-NA-PP1 crossed the blood-brain barrier and reached high enough concentrations in the brain to inhibit AS-PKCε. 1-NA-PP1 prolonged the ataxic and hypnotic effects of ethanol and reduced ethanol consumption by AS-PKCε mice. These effects of 1-NA-PP1 were not observed in wild type mice lacking the AS-PKCε mutation. These results suggest that compounds that inhibit the catalytic activity of PKCε could be useful in reducing ethanol consumption.[3] \n\nPharmacokinetic analyses indicated that 1-NA-PP1 is rapidly and readily detected in the plasma and brain after parenteral administration. We also detected significant amounts of 1-NA-PP1 in the brain after chronic oral administration. 1-NA-PP1 inhibited phosphorylation of the GABAA γ2 subunit at S327 in mouse striatum, indicating that 1-NA-PP1 is able to inhibit PKCε-mediated phosphorylation in vivo. We had previously found that GABAA γ2-S(P)327 immunoreactivity is reduced by 60 ±6% in the frontal cortex of Prkce−/− mice (Qi et al., 2007), and phosphatase treatment did not further reduce this residual immunoreactivity, indicating that the antibody also detects dephosphorylated protein. Therefore, a 60% reduction in GABAA γ2-S(P)327 immunoreactivity represents 100% reduction in phosphorylation at this site. Based on these results, we conclude that intraperitoneal administration of 25mg/kg 1-NA-PP1 reduced PKCε-mediated phosphorylation of GABAA γ2 in AS-PKCε mice by approximately 50%.\n[3] \n1-NA-PP1 reduced ethanol consumption in a reversible manner, without significantly reducing alcohol preference at either of the doses tested. Although water intake was not significantly altered, there was some variability in water intake that may have masked a significant reduction in ethanol preference. At the 30mg/kg dose, there was a trend towards reduced water consumption that was not statistically significant. Saccharin, but not quinine consumption, was significantly reduced at the 30mg/kg dose of 1-NA-PP1. This result is different from what was observed in Prkce−/− mice (Hodge et al., 1999), which showed no deficit in saccharin consumption. It is possible that at the 30mg/kg dose, 1-NA-PP1 reduced ethanol consumption by altering the perception of taste for sweet substances, or by effects on brain reward mechanisms or fluid intake. Of note, a reduction in saccharin and sucrose intake has been observed for naltrexone, which is FDA approved to treat alcohol use disorder (Czachowski and Delory, 2009, Ripley et al., 2015).[3] \n\nBaseline ethanol consumption by wild type C57BL/6NTac mice was much lower than by AS-PKCε mice even though both are on a C57BL/6NTac background. This difference in ethanol consumption could be due to differences in rearing environments and to genetic drift in our AS-PKCε colony from inbreeding. Hence, in addition to the C57BL/6NTac strain, we decided to examine the effects of 1-NA-PP1 on ethanol consumption in C57BL/6J mice, which display high intake and preference for alcohol. Importantly, 1-NA-PP1 did not reduce ethanol drinking in either strain of wild type mice, which both lack the AS-PKCε mutation, indicating that the effects of 1-NA-PP1 on ethanol consumption are specific for AS-PKCε.[3] \n\nOur previous molecular studies suggested that PKCε mediates its effects on ethanol-related behaviors by reducing inhibitory GABA neurotransmission through actions at GABAA receptors. We have identified two substrates of PKCε that could contribute to decreased GABAA receptor function: the GABAA γ2 subunit, which when phosphorylated at S327 shows a reduced response to the positive allosteric effects of benzodiazepines and ethanol (Qi et al., 2007), and the N-ethylmaleimide sensitive factor, which when phosphorylated at S460 and T461 reduces the number of cell surface GABAA receptors (Chou et al., 2010). It is likely that additional PKCε substrates play a role in regulating GABAA receptor function and behavioral responses to ethanol. The M486A mutation allows AS-PKCε to use bulky ATP analogs such as N6-benzyl-ATP as phosphate donors, while native kinases cannot use such ATP analogs (Bishop et al., 2001, Zhang et al., 2013). ATP analogs with a thiophosphate at the γ-phosphate position can generate a kinase-transferable tag, allowing use of a covalent capture-and-release method to purify tagged peptides from digests of protein mixtures (Hertz et al., 2010, Ultanir et al., 2012). Mass spectrometric analysis of these peptides reveals the identity of the corresponding proteins and the location of the phosphorylation sites. Use of this methodology with tissues from AS-PKCε mice could identify novel substrates of PKCε in the brain that regulate GABAA receptor function and behavioral responses to ethanol in an unbiased manner.[3] \nConclusions\nIn summary, our results demonstrate that specific inhibition of PKCε reduces ethanol consumption and prolongs ethanol intoxication, confirming phenotypes we have observed previously using strategies that reduce PKCε expression in the brain. Our results strengthen the rationale for developing small molecule inhibitors of PKCε catalytic activity as therapeutics to decrease ethanol consumption. In addition, our findings demonstrate the utility of the AS-PKCε mouse as a tool for studying the role of PKCε in behavior and for identifying direct substrates of PKCε. 1. 1-Naphthyl PP1 (1-NA-PP1) HCl belongs to the pyrazolopyrimidine scaffold and acts as a pan-PKD inhibitor with high selectivity for PKD over other kinases (e.g., PKCα, PKCδ, CAMKIIα) [2] 2. The gatekeeper mutation (M659G) in PKD1 creates an enlarged ATP-binding pocket that accommodates the bulky naphthyl group of 1-Naphthyl PP1 (1-NA-PP1) HCl, leading to 12-fold increased sensitivity—this enables use of the inhibitor-kinase pair to dissect PKD-specific signaling [2] 3. 1-Naphthyl PP1 (1-NA-PP1) HCl exerts reversible inhibitory effects on AS-PKCε, as evidenced by the recovery of ethanol consumption in AS-PKCε mice 48 h post-administration [3] 4. The PubChem CID of 1-Naphthyl PP1 (1-NA-PP1) HCl (chemical name: 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine) is 4877 [3] 5. 1-Naphthyl PP1 (1-NA-PP1) HCl does not affect the total expression levels of target proteins (e.g., PKD1, PKCε, GABA_A γ2) but specifically inhibits their phosphorylatioctivation [2, 3] |
| 分子式 |
C19H20CLN5
|
|---|---|
| 分子量 |
353.8486
|
| 精确质量 |
353.141
|
| 元素分析 |
C, 64.49; H, 5.70; Cl, 10.02; N, 19.79
|
| CAS号 |
956025-47-1
|
| 相关CAS号 |
1-Naphthyl PP1;221243-82-9
|
| PubChem CID |
10066681
|
| 外观&性状 |
Light yellow to khaki solid
|
| LogP |
5.366
|
| tPSA |
69.62
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
2
|
| 重原子数目 |
25
|
| 分子复杂度/Complexity |
448
|
| 定义原子立体中心数目 |
0
|
| SMILES |
Cl.N1C(N)=C2C(C3C4C(=CC=CC=4)C=CC=3)=NN(C2=NC=1)C(C)(C)C
|
| InChi Key |
UKLRSYUALPIALU-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C19H19N5.ClH/c1-19(2,3)24-18-15(17(20)21-11-22-18)16(23-24)14-10-6-8-12-7-4-5-9-13(12)14;/h4-11H,1-3H3,(H2,20,21,22);1H
|
| 化学名 |
1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine;hydrochloride
|
| 别名 |
1-NA-PP 1 hydrochloride; 956025-47-1; 1-Naphthyl PP1 hydrochloride; 1-Naphthyl PP1 (hydrochloride); 1-tert-butyl-3-naphthalen-1-ylpyrazolo[3,4-d]pyrimidin-4-amine;hydrochloride; 1-tert-butyl-3-(naphthalen-1-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine hydrochloride; 1-tert-Butyl-3-(naphthalen-1-yl)-1H-pyrazolo-[3,4-d]pyrimidin-4-amine hydrochloride; 1-tert-Butyl-3-(naphthalen-1-yl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine HCl; UKLRSYUALPIALU-UHFFFAOYSA-N; 1-Naphthyl PP1 hydrochloride; 1-Naphthyl PP1 (hydrochloride)
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中,避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~71 mg/mL (~200.7 mM)
Ethanol: ~71 mg/mL (~200.7 mM) |
|---|---|
| 溶解度 (体内实验) |
注意: 如下所列的是一些常用的体内动物实验溶解配方,主要用于溶解难溶或不溶于水的产品(水溶度<1 mg/mL)。 建议您先取少量样品进行尝试,如该配方可行,再根据实验需求增加样品量。
注射用配方
注射用配方1: DMSO : Tween 80: Saline = 10 : 5 : 85 (如: 100 μL DMSO → 50 μL Tween 80 → 850 μL Saline)(IP/IV/IM/SC等) *生理盐水/Saline的制备:将0.9g氯化钠/NaCl溶解在100 mL ddH ₂ O中,得到澄清溶液。 注射用配方 2: DMSO : PEG300 :Tween 80 : Saline = 10 : 40 : 5 : 45 (如: 100 μL DMSO → 400 μL PEG300 → 50 μL Tween 80 → 450 μL Saline) 注射用配方 3: DMSO : Corn oil = 10 : 90 (如: 100 μL DMSO → 900 μL Corn oil) 示例: 以注射用配方 3 (DMSO : Corn oil = 10 : 90) 为例说明, 如果要配制 1 mL 2.5 mg/mL的工作液, 您可以取 100 μL 25 mg/mL 澄清的 DMSO 储备液,加到 900 μL Corn oil/玉米油中, 混合均匀。 View More
注射用配方 4: DMSO : 20% SBE-β-CD in Saline = 10 : 90 [如:100 μL DMSO → 900 μL (20% SBE-β-CD in Saline)] 口服配方
口服配方 1: 悬浮于0.5% CMC Na (羧甲基纤维素钠) 口服配方 2: 悬浮于0.5% Carboxymethyl cellulose (羧甲基纤维素) 示例: 以口服配方 1 (悬浮于 0.5% CMC Na)为例说明, 如果要配制 100 mL 2.5 mg/mL 的工作液, 您可以先取0.5g CMC Na并将其溶解于100mL ddH2O中,得到0.5%CMC-Na澄清溶液;然后将250 mg待测化合物加到100 mL前述 0.5%CMC Na溶液中,得到悬浮液。 View More
口服配方 3: 溶解于 PEG400 (聚乙二醇400) 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8261 mL | 14.1303 mL | 28.2606 mL | |
| 5 mM | 0.5652 mL | 2.8261 mL | 5.6521 mL | |
| 10 mM | 0.2826 mL | 1.4130 mL | 2.8261 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。