| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg | |||
| Other Sizes |
| 靶点 |
FAP (fibroblast activation protein)
|
|---|---|
| 体外研究 (In Vitro) |
FAPI框架的化学修饰导致体外FAP结合增加[2]
为了确定放射性示踪剂的fap结合亲和力(补充表1),使用表达fap的人HT-1080细胞进行了放射性配体结合试验。为了弥补FAP表达率的变化,并允许与导联结构进行直接比较,所有实验都与FAPI-04并行进行。所有化合物都显示出与人FAP的强结合,在孵育1和4小时后的结合值等于或高于FAPI-04(图1)。除FAPI-38外,所有化合物的内化率与FAPI-04相当(24小时后内化率为63.1%;FAPI-04, 97.1%;虽然大多数衍生物在24小时后显示出比FAPI-04更高的结合值,但化合物FAPI-38、-39、-40和-41从表达fap的细胞中被清除的速度要快得多,因此不考虑进行更详细的表征。与FAPI-04类似,所有化合物与结构相关的膜蛋白CD26的结合都可以忽略不计(数据未显示)。 |
| 体内研究 (In Vivo) |
FAPI-4(静脉注射;每只小鼠 30 nmol;一次)在 BALB/c nu/nu 小鼠中表现出优异的肿瘤食物效应 [2]。
成纤维细胞激活蛋白(FAP)在几种肿瘤实体的癌症相关成纤维细胞中过度表达。最近开发的以喹啉为基础的PET示踪剂作为FAP抑制剂(fapi)在临床前和一些临床病例中显示出了令人鼓舞的结果。因此,这些示踪剂目前在我院应用,以修正癌症患者面临标准检查限制的诊断。在这里,我们分析了这类新型PET放射性药物的2个成员的组织生物分布和初步剂量学。方法:采用QDOSE剂量测定软件套装,对2例示踪剂注射后0.2、1和3 h检测的患者进行68Ga-FAPI-2和68Ga-FAPI-4的剂量学初步估计。在注射68Ga-FAPI-2 (n = 25)或68Ga-FAPI-4 (n = 25)后1小时对肿瘤患者进行进一步的PET/CT扫描;6例患者进行了个体内相关的18F-FDG扫描(注射后1小时)。对于16个器官的正常组织,在实质中放置一个2厘米的感兴趣的球体体积;对于肿瘤病变,使用阈值分割感兴趣体积来量化SUVmean和SUVmax。结果:与18F-FDG, 68Ga-DOTATATE和68Ga-PSMA-11的文献值相似,200 MBq的68Ga-FAPI-2或68Ga-FAPI-4的检测相当于大约3-4 mSv的等效剂量。经肾脏快速清除后,正常器官示踪剂摄取较低,在注射后10分钟至3小时之间变化极小。68Ga-FAPI-2注射后1 ~ 3 h的肿瘤摄取减少75%,而68Ga-FAPI-4注射后肿瘤滞留时间延长(25%洗脱)。关于肿瘤与背景比,注射后1小时,两种68Ga-FAPI示踪剂表现相同。与18F-FDG相比,肿瘤摄取几乎相等(平均SUVmax, 18F-FDG为7.41,68Ga-FAPI-2为7.37;无统计学意义);68Ga-FAPI在脑(11.01 vs. 0.32)、肝脏(2.77 vs. 1.69)和口腔/咽粘膜(4.88 vs. 2.57)的背景摄取显著降低。其他器官在18F-FDG和68Ga-FAPI之间没有相关性差异。结论:FAPI PET/CT是一种新的肿瘤诊断方法。与18F-FDG相比,在检查前不需要节食或禁食,在使用示踪剂几分钟后就可以开始图像采集。肿瘤与背景对比度等于甚至优于18F-FDG[1]。 |
| 细胞实验 |
细胞培养[1]
转染人FAP基因的HT-1080细胞,以及小鼠FAP和cd26转染的人胚胎肾细胞,在含有10%胎牛血清的Dulbecco修饰Eagle培养基中,在37°C/5%二氧化碳条件下培养。 对于放射性配体结合研究,将细胞接种于6孔板中,培养48 h,最终汇合率约为80%-90%(每孔120 - 20万个细胞)。用1 mL不含胎牛血清的新鲜培养基代替培养基。放射性标记的化合物添加到细胞培养,培养不同的间隔10分钟至24小时。竞争实验由同时暴露在无标号(10−5到10−10米)和放射性标记的化合物60分钟。细胞流出决心孵化后的细胞示踪60分钟。此后,放射性介质被移除,细胞被洗和孵化与非放射性介质1,2,4,24 h。在所有的实验中,细胞用1ml pH为7.4的磷酸盐缓冲盐水洗涤两次,然后用1.4 mL裂解缓冲液(0.3 m NaOH, 0.2%十二烷基硫酸钠)裂解。在γ-计数器中测定放射性,归一化到100万个细胞,并以施加剂量的百分比计算。每个实验进行3次,每个独立实验进行3次重复。 |
| 动物实验 |
Animal/Disease Models: 8weeks old BALB/c nu/nu (nude) mice were inoculated with HT-1080-FAP cells [2].
Doses: 30 nmol per mouse. Route of Administration: intravenous (iv) (iv)injection; 30 nmol per mouse; one time. Experimental Results: Display demonstrated higher overall tumor uptake (9.44% ID/g 4 hrs (hrs (hours)) after injection). |
| 药代性质 (ADME/PK) |
Improvement of Pharmacokinetics and Image Contrast for PET[2]
To assess a potential increase in tumor retention and to evaluate their pharmacokinetic behavior, the most promising candidates were analyzed in vivo. To this end, small-animal PET imaging was performed on HT-1080-FAP xenografted mice. All compounds demonstrated rapid tumor accumulation with overall low background activity and predominantly renal elimination (Supplemental Fig. 4). Tumor uptake was highest for FAPI-55 (SUVmax of 1.8 after 60 min and 1.7 after 120 min), followed by FAPI-36 (1.5 after 60 min and 1.3 after 120 min) and FAPI-21 (1.3 after both 60 and 120 min) (Fig. 2, Supplemental Fig. 5). Because the absolute uptake values allow only a limited comparison of the radiotracers, AUCs were calculated from the time–activity curves, representing the accumulated radioactivity within the interval up to 2 h after injection. As shown in Table 1, 7 of 10 compounds demonstrated higher tumor uptake than that for FAPI-04, headed by FAPI-21, -36, -46, and -55. Yet, FAPI-36 showed a prolonged systemic circulation, resulting in unfavorable tumor-to-blood ratios and a poorer image contrast than that for FAPI-04 (Supplemental Fig. 4). Although the tumor-to-blood and tumor-to-liver ratios for FAPI-35 were comparable to those for FAPI-04, the tumor-to-muscle ratio was slightly improved (Fig. 3). FAPI-21 and -55 demonstrated higher accumulation in liver and muscle tissue than did FAPI-04. From all tested compounds, FAPI-46 displayed the highest tumor-to-blood, tumor-to-muscle, and tumor-to-liver ratios. On the basis of the observations in the imaging studies, FAPI-21, -35, -46, and -55 were selected for a more detailed characterization in biodistribution studies using 177Lu-labeled radiotracers. As shown in Figure 4, all compounds demonstrated robust tumor accumulation with overall low uptake into healthy tissue. Moderate radioactivity (1.8–3.5 %ID/g 1 h after injection) was measured only in the kidneys, because of the predominantly renal elimination of the radiotracers, with activity mostly in the renal calyx system. In comparison to FAPI-04, FAPI-21 and -46 demonstrated higher tumor accumulation 1 and 4 h after injection. Although all other compounds displayed their highest intratumoral radioactivity 1 h after injection, tumor uptake was increasing even from 1 to 4 h for FAPI-21. In addition, FAPI-21 revealed the highest tumor retention 24 h after injection (6.03 ± 0.68 %ID/g), followed by FAPI-35 (2.47 ± 0.23 %ID/g) and -46 (2.29 ± 0.16 %ID/g), featuring uptake rates similar to FAPI-04 (2.86 ± 0.31 %ID/g). Accordingly, 64% of the maximum tumor activity was still present 24 h after injection for FAPI-21, followed by FAPI-35 (37%), FAPI-46, and FAPI-55 (almost 20% each). In comparison to FAPI-04, radioactivity levels in the blood were equal or marginally higher at all specified times, except for FAPI-55, which demonstrated the highest blood activities of all compounds up to 6 h after injection. However, blood activity was decreasing steadily, reaching values similar to FAPI-04 after 24 h. All derivatives demonstrated an increased liver uptake as compared with FAPI-04, except for FAPI-46, which displayed comparable activities up to 6 h after injection but narrowed to lower levels in the course of 24 h. The renal activity of the compounds was comparable for FAPI-04, -21, and -35 but significantly reduced for FAPI-46 and -55 at all specified times. A comparison of AUCs, determined from the time–activity curves from 1 to 24 h after injection, revealed the highest overall tumor uptake to be for FAPI-21, followed by FAPI-46 (Table 2). A calculation of tumor-to-organ ratios, based on the overall AUCs, evinced a general improvement in pharmacokinetics for FAPI-21 and -46 and no considerable change for any of the other radiotracers, except for FAPI-35 (Fig. 5, Supplemental Table 3). Notably, FAPI-46 displayed substantially improved ratios of tumor to liver, kidney, and brain uptake.[2] Biodistribution[1] The 2 patients examined 10 min to 3 h after injection demonstrated that both FAPI tracers rapidly reached their stable physiologic biodistribution. In normal tissue, changes between 10 min and 3 h after injection were minimal. Tumor uptake declined by a mean of 75% from 1 h to 3 h after injection using 68Ga-FAPI-2; less washout, only 25% (mean), between 1 h and 3 h after injection (i.e., longer tumor retention) was observed with 68Ga-FAPI-4 (Fig. 2, bottom). However, at 1 h after injection (the time point also chosen for comparison to 18F-FDG), both 68Ga-FAPI tracers performed equally with regard to tumor-to-background ratios.[1] The quantitative tumor uptake of FAPI PET was similar to that of the current oncologic PET standard of reference, 18F-FDG (average SUVmax, 7.41 for 18F-FDG and 7.37 for 68Ga-FAPI-2; not statistically significant). In pancreatic, esophageal, lung, head and neck, and colorectal cancer, the quantitative tumor uptake was noninferior to that of 18F-FDG. In contrast, dedifferentiated thyroid cancer with flip-flop uptake of 18F-FDG was not accumulating 68Ga-FAPI (Fig. 3). Regarding background activity, the average SUVmax of 68Ga-FAPI-2 was significantly lower in brain (0.32 vs. 11.01), liver (1.69 vs. 2.77), and oral/pharyngeal mucosa (2.57 vs. 4.88), thus improving the contrast ratios for liver metastases of pancreatic and colorectal cancer and delineation of the esophageal cancer (Fig. 3). For all other organs, 68Ga-FAPI-2 presented no significant difference from 18F-FDG (Fig. 4A). |
| 参考文献 |
|
| 其他信息 |
Cancer-associated fibroblasts constitute a vital subpopulation of the tumor stroma and are present in more than 90% of epithelial carcinomas. The overexpression of the serine protease fibroblast activation protein (FAP) allows a selective targeting of a variety of tumors by inhibitor-based radiopharmaceuticals (FAPIs). Of these compounds, FAPI-04 has been recently introduced as a theranostic radiotracer and demonstrated high uptake into different FAP-positive tumors in cancer patients. To enable the delivery of higher doses, thereby improving the outcome of a therapeutic application, several FAPI variants were designed to further increase tumor uptake and retention of these tracers. Methods: Novel quinoline-based radiotracers were synthesized by organic chemistry and evaluated in radioligand binding assays using FAP-expressing HT-1080 cells. Depending on their in vitro performance, small-animal PET imaging and biodistribution studies were performed on HT-1080-FAP tumor-bearing mice. The most promising compounds were used for clinical PET imaging in 8 cancer patients. Results: Compared with FAPI-04, 11 of 15 FAPI derivatives showed improved FAP binding in vitro. Of these, 7 compounds demonstrated increased tumor uptake in tumor-bearing mice. Moreover, tumor-to-normal-organ ratios were improved for most of the compounds, resulting in images with higher contrast. Notably two of the radiotracers, FAPI-21 and -46, displayed substantially improved ratios of tumor to blood, liver, muscle, and intestinal uptake. A first diagnostic application in cancer patients revealed high intratumoral uptake of both radiotracers already 10 min after administration but a higher uptake in oral mucosa, salivary glands, and thyroid for FAPI-21. Conclusion: Chemical modification of the FAPI framework enabled enhanced FAP binding and improved pharmacokinetics in most of the derivatives, resulting in high-contrast images. Moreover, higher doses of radioactivity can be delivered while minimizing damage to healthy tissue, which may improve therapeutic outcome.[2]
|
| 分子式 |
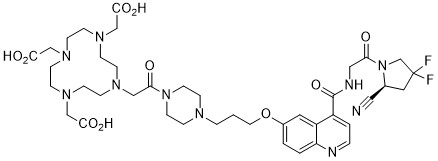
C40H54F2N10O10
|
|---|---|
| 分子量 |
872.93
|
| 精确质量 |
872.4
|
| 元素分析 |
C, 55.04; H, 6.24; F, 4.35; N, 16.05; O, 18.33
|
| CAS号 |
2374782-02-0
|
| PubChem CID |
138454803
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.46±0.1 g/cm3(Predicted)
|
| 沸点 |
1144.1±65.0 °C(Predicted)
|
| LogP |
-6.8
|
| tPSA |
244
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
19
|
| 可旋转键数目(RBC) |
16
|
| 重原子数目 |
62
|
| 分子复杂度/Complexity |
1580
|
| 定义原子立体中心数目 |
1
|
| SMILES |
FC1(C([H])([H])N(C(C([H])([H])N([H])C(C2C([H])=C([H])N=C3C([H])=C([H])C(=C([H])C=23)OC([H])([H])C([H])([H])C([H])([H])N2C([H])([H])C([H])([H])N(C(C([H])([H])N3C([H])([H])C([H])([H])N(C([H])([H])C(=O)O[H])C([H])([H])C([H])([H])N(C([H])([H])C(=O)O[H])C([H])([H])C([H])([H])N(C([H])([H])C(=O)O[H])C([H])([H])C3([H])[H])=O)C([H])([H])C2([H])[H])=O)=O)[C@]([H])(C#N)C1([H])[H])F
|
| InChi Key |
ICWDAESAANBIGG-LJAQVGFWSA-N
|
| InChi Code |
InChI=1S/C40H54F2N10O10/c41-40(42)21-29(22-43)52(28-40)34(53)23-45-39(61)31-4-5-44-33-3-2-30(20-32(31)33)62-19-1-6-46-15-17-51(18-16-46)35(54)24-47-7-9-48(25-36(55)56)11-13-50(27-38(59)60)14-12-49(10-8-47)26-37(57)58/h2-5,20,29H,1,6-19,21,23-28H2,(H,45,61)(H,55,56)(H,57,58)(H,59,60)/t29-/m0/s1
|
| 化学名 |
(S)-2,2',2''-(10-(2-(4-(3-((4-((2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)quinolin-6-yl)oxy)propyl)piperazin-1-yl)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid
|
| 别名 |
FAPI-04; FAPI 04; FAPI04; FAPI-4; FAPI-4; 2374782-02-0; DOTA-fapi-04; (S)-2,2',2''-(10-(2-(4-(3-((4-((2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)carbamoyl)quinolin-6-yl)oxy)propyl)piperazin-1-yl)-2-oxoethyl)-1,4,7,10-tetraazacyclododecane-1,4,7-triyl)triacetic acid; D7X5DKW7N9; FAPI-04; UNII-D7X5DKW7N9; SCHEMBL21257058; FAPI 4; FAPI4;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~114.56 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (2.86 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (2.86 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (2.86 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 4 mg/mL (4.58 mM) in Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.1456 mL | 5.7278 mL | 11.4557 mL | |
| 5 mM | 0.2291 mL | 1.1456 mL | 2.2911 mL | |
| 10 mM | 0.1146 mL | 0.5728 mL | 1.1456 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。