| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| Other Sizes |
| 靶点 |
IC50: 75 nM (long chain 3-ketoyl coenzyme A thiolase)[2] β-oxidation[2] Autophagy[3] 3-hydroxyacyl-CoA dehydrogenase (HADHA)[4]
|
|---|---|
| 体外研究 (In Vitro) |
曲美他嗪(1–100 μM;24 小时;HUVEC)以剂量依赖性方式提高已经历氧化损伤的 HUVEC 的活力 [1]。
|
| 体内研究 (In Vivo) |
在小鼠 ICES 测试中,剂量为 10 和 20 mg/kg 的曲美他嗪(5-20 mg/kg;PO;1 小时;瑞士白化雄性小鼠)显着升高癫痫阈值电流 [5]。
|
| 酶活实验 |
曲美他嗪在任何有氧灌注条件下对心肌耗氧量或心脏功都没有影响。在用5 mmol/L葡萄糖和0.4 mmol/L棕榈酸灌注的心脏中,曲美他嗪将棕榈酸氧化速率从488+/-24降低到408+/-15 nmol x g干重(-1)x分钟(-1)(P<0.05),而将葡萄糖氧化速率从1889+/-119增加到2378+/-166 nmol x g干重(-1)x分钟。在低流量缺血的心脏中,曲美他嗪导致葡萄糖氧化率增加210%。在有氧心脏和缺血性心脏中,曲美他嗪对糖酵解速率没有影响。曲美他嗪对葡萄糖氧化的影响伴随着丙酮酸脱氢酶活性形式增加37%,丙酮酸脱氢酶是葡萄糖氧化的限速酶。当棕榈酸盐被0.8 mmol/L辛酸盐或1.6 mmol/L丁酸盐取代时,未观察到曲美他嗪对糖酵解、葡萄糖氧化、脂肪酸氧化或活性丙酮酸脱氢酶的影响,这表明曲美他啶直接抑制长链脂肪酸氧化。脂肪酸氧化的这种减少伴随着参与脂肪酸β氧化的最后一种酶的长链异构体3-酮酰基辅酶a(CoA)硫解酶活性(IC(50)为75nmol/L)的显著降低。相反,需要超过10和100微mol/L的曲美他嗪浓度来分别抑制3-酮酰基辅酶A硫解酶的中链和短链形式。先前的研究表明,抑制脂肪酸氧化和刺激葡萄糖氧化可以保护缺血性心脏。因此,我们的数据表明,曲美他嗪的抗心脏病作用可能是因为长链3-酮酰基辅酶a硫解酶活性受到抑制,从而导致脂肪酸氧化减少和葡萄糖氧化刺激[3]。
|
| 细胞实验 |
细胞活力测定[1]
细胞类型: 人脐静脉内皮细胞 (HUVEC) 测试浓度: 1 μM、10 μM、100 μM 孵化持续时间:24小时 实验结果:增强氧化诱导的受损HUVEC的活力。 |
| 动物实验 |
Animal/Disease Models: Swiss albino male mice (24-35 g)[4]
Doses: 5 mg/kg, 10 mg/kg and 20 mg/kg; 10 mL/kg body weight Route of Administration: Oral administration; 1 hour Experimental Results: In 10 and 20mg/kg doses Dramatically raised the seizure-threshold current in the ICES test. |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
In elderly patients, a 35 mg oral modified release tablet reaches a mean Cmax of 115 µg/L, with a Tmax of 2.0-5.0 hours, and a mean AUC0-12 of 1104 h\*µg/L. In young, healthy patients, the same dose reaches a mean Cmax of 91.2 µg/L, with a Tmax of 2.0-6.0 hours, and an AUC0-12h 720 h\*µg/L. Trimetazidine is 79-84% eliminated in the urine, with 60% as the unchanged parent compound. In a study of 4 healthy subjects, individual metabolites made up 0.01-1.4% of the dose recovered in urine. In the urine, 2-desmethyltrimetazidine made up 0-1.4% of the recovered dose, 3- and 4-desmethyltrimetazidine made up 0.039-0.071% each, N-methyltrimetazidine made up 0.015-0.11%, trimetazidine ketopiperazine made up 0.011-0.4%, N-formyltrimetazidine made up 0.035-0.42%, N-acetyltrimetazidine made up 0.016-0.19%, desmethyl trimetazidine O-sulphate made up 0.01-0.65%, and an unknown metabolite made up0.026-0.67%. The volume of distribution of trimetazidine is 4.8 L/kg. Trimetazidine clearance is strongly correlated with creatinine clearance. In eldery patients with a creatinine clearance of 72 ± 8 mL/min, trimetazidine clearance was 15.69 L/h. In young, healthy patients with a creatinine clearance of 134 ± 18 mL/min, trimetazidine clearance was 25.2 L/h. Metabolism / Metabolites Trimetazidine can be oxidized at the piperazine ring to form trimetazidine ketopiperazine. Trimetazidine can also be N-formylated, N-acetylated, or N-methylated at the piperazine ring to form N-formyltrimetazidine, N-acetyltrimetazidine, and N-methyltrimetazidine respectively. Alternatively, trimetazidine can be demethylated at the 2, 3, or 4 position of the 2,3,4-trimethoxybenzyl moiety to form 2-desmethyltrimetazidine, 3-desmethyltrimetazidine, or 4-desmethyltrimetazidine. The desmethyltrimetazidine metabolites can undergo sulfate conjugation or glucuronidation prior to elimination. Biological Half-Life In young, healthy subjects, the half life of trimetazidine is 7.81 hours. In patients over 65, the half life increases to 11.7 hours. |
| 毒性/毒理 (Toxicokinetics/TK) |
Protein Binding
Trimetazidine is 15% protein bound in plasma. Trimetazidine can bind to human serum albumin. |
| 参考文献 |
|
| 其他信息 |
1-[(2,3,4-trimethoxyphenyl)methyl]piperazine is an aromatic amine.
Trimetazidine is a piperazine derivative indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Trimetazidine has been studied as a treatment for angina pectoris since the late 1960s. Acidic conditions, caused by anaerobic metabolism and fatty acid oxidation, in response to myocardial ischemia, activate sodium-hydrogen and sodium-calcium antiport systems. The increased intracellular calcium decreases contractility. It is hypothesized that trimetazidine inhibits 3-ketoacyl coenzyme A thiolase, which decreases fatty acid oxidation but not glucose metabolism, preventing the acidic conditions that exacerbate ischemic injury. However, evidence for this mechanism is controversial. Trimetazidine is not FDA approved. However, it has been approved in France since 1978. Trimetazidine is an orally available small molecule compound with anti-ischemic, and potential immunomodulating and antineoplastic properties. Although its exact mechanism is not yet fully elucidated, it is postulated that upon administration, trimetazidine selectively inhibits long-chain 3-ketoacyl coenzyme A thiolase (LC 3-KAT), the final enzyme in the free fatty acid (FFA) beta-oxidation pathway. This stimulates glucose oxidation, which requires less oxygen usage and cellular energy than in the beta-oxidation process. This optimizes myocardial energy metabolism and cardiac functioning in an ischemic condition. In cancer cells, the inhibition of fatty acid oxidation (FAO) alters the metabolic processes needed for tumor cell function and proliferation, thereby inducing tumor cell apoptosis. In addition, inhibition of FAO may potentially block the immunosuppressive functions of myeloid-derived suppressor cells (MSDCs), which are thought to promote malignant cell proliferation and migration by inhibiting T-cell function. A vasodilator used in angina of effort or ischemic heart disease. Drug Indication Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Mechanism of Action During myocardial ischemia, anaerobic metabolism takes over, increasing levels of lactic acid. The decreased intracellular pH and increased concentration of protons activates sodium-hydrogen and sodium-calcium antiport systems, raising intracellular calcium concentrations, finally leading to decreased contractility. This injury to the myocardium raises concentrations of catecholamines, which activate hormone sensitive lipase, and increasing fatty acid concentrations in plasma. When the myocardium is repurfused, fatty acid oxidation becomes the dominant form of ATP production, maintaining an acidic pH, and further exacerbating the injury. The mechanism of action of trimetazidine is not fully understood. Trimetazidine may inhibit mitochondrial 3-ketoacyl coenzyme A thiolase, decreasing long chain fatty acid β-oxidation but not glycolysis in the myocardium. The decreased long chain fatty acid β-oxidation is compensated for by increased use of glucose, preventing a lowered myocardial pH, and further decreases in contractility. However, another study suggests that 3-ketoacyl coenzyme A thiolase may not be trimetazidine's target, and that this mechanism may be incorrect. Pharmacodynamics Trimetazidine is indicated for the symptomatic treatment of stable angina pectoris in patients inadequately controlled or intolerant to first line therapies. Patients should be counselled regarding the risk of use with reduced renal or hepatic function, worsening of extrapyramidal symptoms or other movement disorders, and risk of falls. |
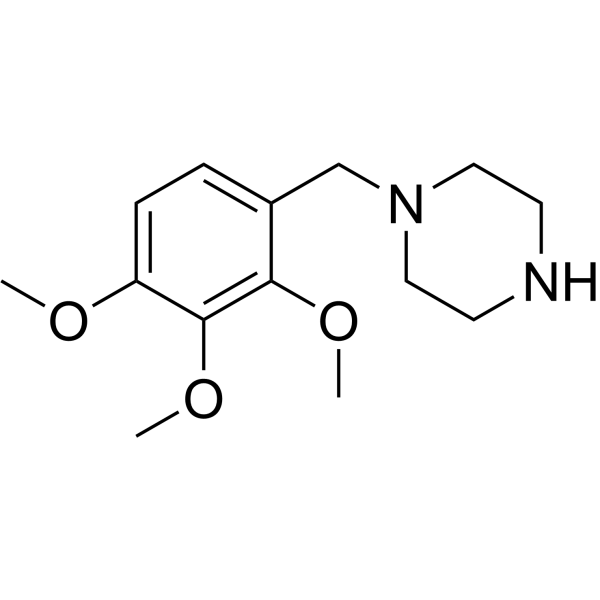
| 分子式 |
C14H22N2O3
|
|---|---|
| 分子量 |
266.34
|
| 精确质量 |
274.213
|
| 元素分析 |
C, 63.13; H, 8.33; N, 10.52; O, 18.02
|
| CAS号 |
5011-34-7
|
| 相关CAS号 |
Trimetazidine dihydrochloride;13171-25-0
|
| PubChem CID |
21109
|
| 外观&性状 |
White to off-white ointment
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
364.0±37.0 °C at 760 mmHg
|
| 熔点 |
200 - 205ºC
|
| 闪点 |
174.0±26.5 °C
|
| 蒸汽压 |
0.0±0.8 mmHg at 25°C
|
| 折射率 |
1.524
|
| LogP |
0.8
|
| tPSA |
42.96
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
19
|
| 分子复杂度/Complexity |
259
|
| 定义原子立体中心数目 |
0
|
| SMILES |
COC1=C(C(=C(C=C1)CN2CCNCC2)OC)OC
|
| InChi Key |
UHWVSEOVJBQKBE-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C14H22N2O3/c1-17-12-5-4-11(13(18-2)14(12)19-3)10-16-8-6-15-7-9-16/h4-5,15H,6-10H2,1-3H3
|
| 化学名 |
1-[(2,3,4-trimethoxyphenyl)methyl]piperazine
|
| 别名 |
TRIMETAZIDINE; 5011-34-7; 1-(2,3,4-Trimethoxybenzyl)piperazine; 1-[(2,3,4-trimethoxyphenyl)methyl]piperazine; 1-(2,3,4-Trimethoxy-benzyl)-piperazine; Piperazine, 1-((2,3,4-trimethoxyphenyl)methyl)-; N9A0A0R9S8; Trimetazidine (INN);
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 本产品在运输和储存过程中需避光。 (2). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ≥ 125 mg/mL (469.32 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.08 mg/mL (7.81 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 20.8 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (7.81 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (7.81 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.7546 mL | 18.7730 mL | 37.5460 mL | |
| 5 mM | 0.7509 mL | 3.7546 mL | 7.5092 mL | |
| 10 mM | 0.3755 mL | 1.8773 mL | 3.7546 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。