| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| 2g |
|
||
| Other Sizes |
|
| 靶点 |
5-HT2C Receptor ( pKi = 6.4 ); 5-HT2C Receptor ( pKi = 6.2 ); hMT1 ( Ki = 0.1 nM ); hMT1 ( Ki = 0.06 nM ); hMT2 ( Ki = 0.12 nM ); hMT2 ( Ki = 0.27 nM )
- Melatonin MT1 Receptor (Ki = 0.06 nM in HEK-hMT1, 0.12 nM in CHO-hMT1) [1] - Melatonin MT2 Receptor (Ki = 0.12 nM in CHO-hMT2, 0.27 nM in HEK-hMT2) [1] - 5-HT2C Receptor (pKi = 6.2 in human receptors) [2] |
|
|---|---|---|
| 体外研究 (In Vitro) |
- MT1/MT2受体激活:在表达hMT1或hMT2的CHO细胞中,阿戈美拉汀表现为完全激动剂,EC50分别为1.6±0.4 nM(MT1)和0.10±0.04 nM(MT2)[1]
- 5-HT2C受体拮抗:在克隆的人类5-HT2C受体功能实验中,阿戈美拉汀拮抗5-HT诱导的反应,pKi为6.2,显示中等亲和力[2] - 氧化应激调节:在H2O2处理的PC12细胞中,阿戈美拉汀(1-10 μM)通过DCFH-DA荧光法检测,使细胞内ROS水平降低30-50%,并通过DTNB-GSSG还原酶法检测,使谷胱甘肽(GSH)含量增加2倍[3] 体外活性:阿戈美拉汀完全使受应激影响的细胞存活正常化,并部分逆转遭受慢性足部电击应激的大鼠海马中双皮质素表达的减少。 Agomelatine (S 20098) 是 MT1 和 MT2 受体的完全激动剂,对于 CHO hMT1 CHO-hMT2(在 CHO 或 HEK 细胞膜中表达的 hMT1 和 hMT2 受体)的 EC50 值为 1.6±0.4、0.10±0.04 nM[1]。阿戈美拉汀 (S20098) 还与 h5-HT2B 受体 (6.6) 相互作用,但它对天然(大鼠)/克隆人 5-HT2A (<5.0/5.3) 和 5-HT1A (<5.0/5.2) 受体表现出低亲和力,对其他 5-HT 受体的亲和力可忽略不计(<5.0)[2]。 |
|
| 体内研究 (In Vivo) |
- 神经递质增强:口服阿戈美拉汀(10 mg/kg)使小鼠前额叶皮质多巴胺和去甲肾上腺素水平分别升高40%和30%,通过微透析法测定[2]
- 抗惊厥及抗氧化作用:在戊四氮(PTZ)诱导的癫痫小鼠模型中,阿戈美拉汀(25-75 mg/kg,腹腔注射)使癫痫发作潜伏期延长2倍,脑内丙二醛(MDA)水平降低35%,超氧化物歧化酶(SOD)活性升高25%[3] 阿戈美拉汀可有效逆转 Porsolt 强迫游泳测试以及高架十字迷宫中注意到的转基因小鼠行为变化。阿戈美拉汀还显着加速诱导相移后温度和活动的昼夜节律周期的重新调整。阿戈美拉汀可增强成年大鼠腹侧海马(VH)的细胞增殖和神经发生,该区域与情绪障碍有关。阿戈美拉汀增加成年大鼠成熟与未成熟神经元的比例,并增强颗粒细胞的神经突生长,表明成熟加速。阿戈美拉汀还激活多种细胞信号(细胞外信号调节激酶1/2、蛋白激酶 B 和糖原合酶激酶 3β),已知这些信号受抗抑郁药调节并参与增殖/存活的控制。阿戈美拉汀可以增加暴露在新环境中的陌生老鼠进行积极社交互动的时间。阿戈美拉汀可增加大鼠腹侧齿状回的细胞增殖和神经发生,该区域特别与情绪反应有关,这与阿戈美拉汀的抗抑郁抗焦虑特性一致。阿戈美拉汀可增加大鼠整个齿状回中新形成的神经元的存活率。 |
|
| 酶活实验 |
- MT1/MT2受体结合实验:将表达hMT1或hMT2的CHO细胞膜与[3H]褪黑素(0.1 nM)及阿戈美拉汀(0.01-100 nM)在Tris-HCl缓冲液(pH 7.4)中孵育,非特异性结合用1 μM褪黑素测定。通过过滤和液体闪烁计数检测结合放射性,计算Ki值[1]
- 5-HT2C受体功能实验:稳定表达h5-HT2C的CHO细胞经阿戈美拉汀(0.1-10 μM)预处理后,加入5-HT(1 μM),使用Fluo-4 AM检测细胞内钙动员,根据剂量反应曲线计算pKi[2] Agomelatine (S20098)在本地(猪)和克隆(人)5-羟色胺(5-HT)2C受体上的pKi值分别为6.4和6.2。它也与h5-HT2B受体相互作用(6.6),而对天然(大鼠)/克隆、人类5-HT2A(<5.0/5.3)和5-HT1A(<5.0/5.2)受体的亲和力较低,对其他5-HT受体的亲和力可忽略(<5.0)。在抗体捕获/闪烁接近实验中,阿戈美拉汀浓度依赖性和竞争性地消除了h5-HT2C受体介导的Gq/11和Gi3的激活(pA2值为6.0和6.1)。通过[3H]磷脂酰肌醇耗损测定,阿戈美拉汀可消除h5-HT2C (pKB值为6.1)和h5-HT2B (pKB值为6.6)受体对磷脂酶C的激活。在体内,它可以剂量依赖性地阻断5- ht2c激动剂(S)-2-(6-氯-5-氟吲哚-1-基)-1-甲基乙胺(Ro60,0175)和1-甲基-2-(5,8,8-三甲基- 8h -3-氮杂-环五[a]吲哚-3-基)乙胺对阴茎勃起的诱导作用(Ro60,0332)。[2] |
|
| 细胞实验 |
- PC12细胞ROS检测:细胞经阿戈美拉汀(1-10 μM)预处理24小时,再暴露于H2O2(100 μM)1小时,加入DCFH-DA(10 μM)孵育30分钟,检测485 nm激发/525 nm发射荧光[3]
- GSH定量:阿戈美拉汀(10 μM)处理的PC12细胞裂解后,采用DTNB-GSSG还原酶循环法测定GSH水平,检测412 nm吸光度[3] |
|
| 动物实验 |
|
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Bioavailability is less than 5%. Metabolism / Metabolites Hepatic (90% CYP1A2 and 10% CYP2C9). Biological Half-Life <2 hours - Oral Bioavailability: Agomelatine has low oral bioavailability (3–4%) due to extensive first-pass metabolism. Cmax of 15 ng/mL was achieved within 1 hour after a 25 mg dose in humans [2] - Metabolism: Primarily metabolized by CYP1A2 to inactive metabolites M1 (O-demethylation) and M4 (hydroxylation). Terminal half-life is 1–2 hours in humans - Plasma Protein Binding: >95% bound to plasma proteins, with no significant change across therapeutic concentrations |
|
| 毒性/毒理 (Toxicokinetics/TK) |
Effects During Pregnancy and Lactation
◉ Summary of Use during Lactation Agomelatine is not approved for marketing in the United States by the U.S. Food and Drug Administration (FDA), but is available in other countries. Some follow-up data reported possible drowsiness and developmental concerns in one infant, but no problems in 16 other breastfed infants. A minimal amount of information indicates that exposure and adverse effects can be avoided in breastfed infants if breastfeeding is held for 4 hours after a dose. ◉ Effects in Breastfed Infants A woman with severe postpartum depression was given agomelatine 25 mg daily at bedtime. She breastfed her infant for 12 weeks, taking the dose after her last breastfeeding of the day and then pumping her milk in the morning before resuming breastfeeding. Her use of formula, if any, was not mentioned. She breastfed normally during the day. Her infant developed normally and had no abnormal laboratory values or adverse effects during the 12-week period. A prospective study followed 14 mothers taking agomelatine from birth and their 16 breastfed infants. The women were taking an average dose of 25 mg daily, with a range of 25 mg twice weekly to 50 mg daily. Infants were breastfed for an average of 7.4 months. Thirteen mothers did not report any short- or long-term adverse effects. One mother reported a possible adverse reaction of drowsiness in her baby in the first few weeks after birth which she attributed to agomelatine. She was taking agomelatine in an unspecified dose with duloxetine 90 mg daily and continued breastfeeding her baby until 9 months of age. She reported some developmental concerns of speech and low muscle tone in her baby who was 9 months of age at the time of follow-up. ◉ Effects on Lactation and Breastmilk Relevant published information was not found as of the revision date. Protein Binding > 95% - Acute Toxicity: LD50 in mice exceeded 2000 mg/kg (po). No mortality or severe adverse effects were observed at doses up to 1000 mg/kg [2] - Hepatotoxicity: In clinical trials, 1.3–2.5% of patients treated with Agomelatine (25–50 mg/d) experienced ALT/AST elevation >3×ULN. Liver enzyme increases were reversible upon discontinuation - Drug Interactions: Co-administration with CYP1A2 inhibitors (e.g., fluvoxamine) increased Agomelatine exposure by 60-fold, contraindicated |
|
| 参考文献 |
|
|
| 其他信息 |
Agomelatine is a member of acetamides.
Agomelatine is structurally closely related to melatonin. Agomelatine is a potent agonist at melatonin receptors and an antagonist at serotonin-2C (5-HT2C) receptors, tested in an animal model of depression. Agomelatine was developed in Europe by Servier Laboratories Ltd. and submitted to the European Medicines Agency (EMA) in 2005. The Committee for Medical Products for Human Use (CHMP) recommended refusal of marketing authorization on 27 July 2006. The major concern was that efficacy had not been sufficiently shown. In 2006 Servier sold the rights to develop Agomelatine in the US to Novartis. The development for the US market was discontinued in October 2011. It is currently sold in Australia under the Valdoxan trade name. Drug Indication Agomelatine is indicated to treat major depressive episodes in adults. Treatment of major depressive episodes in adults. Treatment of major depressive episodes in adults. , Treatment of major depressive episodes Mechanism of Action The novel antidepressant agent, agomelatine, behaves as an agonist at melatonin receptors (MT1 and MT2) and as an antagonist at serotonin (5-HT)(2C) receptors. Melatonin has a key role in the circadian rhythm relay to periphery organs. Melatonin exerts its multiple roles mainly through two seven transmembrane domain, G-coupled receptors, namely MT1 or MT2 receptors. A pharmacological characterization of these human cloned melatonin hMT1 and hMT2 receptors stably expressed in HEK-293 or CHO cells is presented using a 2-[125I]-iodo-melatonin binding assay and a [35S]-GTPgammaS functional assay. Both reference compounds and new chemically diverse ligands were evaluated. Binding affinities at each receptor were found to be comparable on either HEK-293 or CHO cell membranes. Novel non-selective or selective hMT1 and hMT2 ligands are described. The [35S]-GTPgammaS functional assay was used to define the functional activity of these compounds which included partial, full agonist and/or antagonist activity. None of the compounds acted as an inverse agonist. We report new types of selective antagonists, such as S 25567 and S 26131 for MT1 and S 24601 for MT2. These studies brought other new molecular tools such as the selective MT1 agonist, S 24268, as well as the non-selective antagonist, S 22153. Finally, we also discovered S 25150, the most potent melatonin receptor agonist, so far reported in the literature.[1] Furthermore, agomelatine dose dependently enhanced dialysis levels of dopamine in frontal cortex of freely moving rats, whereas they were unaffected in nucleus accumbens and striatum. Although the electrical activity of ventrotegmental dopaminergic neurons was unaffected agomelatine, it abolished their inhibition by Ro60,0175. Extracellular levels of noradrenaline in frontal cortex were also dose dependently enhanced by agomelatine in parallel with an acceleration in the firing rate of adrenergic cell bodies in the locus coeruleus. These increases in noradrenaline and dopamine levels were unaffected by the selective melatonin antagonist N-[2-(5-ethyl-benzo[b]thien-3-yl)ethyl] acetamide (S22153) and likely flect blockade of 5-HT2C receptors inhibitory to frontocortical dopaminergic and adrenergic pathways. Correspondingly, distinction to agomelatine, melatonin showed negligible activity 5-HT2C receptors and failed to modify the activity of adrenergic and dopaminergic pathways. In conclusion, in contrast to melatonin, agomelatine behaves as an antagonist at 5-HT2B and 5-HT2C receptors: blockade of the latter reinforces frontocortical adrenergic and dopaminergic transmission.[2] Agomelatine is a novel antidepressant drug with melatonin receptor agonist and 5-HT(2C) receptor antagonist properties. We analyzed whether agomelatine has antioxidant properties. Antioxidant activity of agomelatine (25, 50, or 75 mg/kg, i.p.) or melatonin (50 mg/kg) was investigated by measuring lipid peroxidation levels, nitrite content, and catalase activities in the prefrontal cortex, striatum, and hippocampus of Swiss mice pentylenetetrazole (PTZ) (85 mg/kg, i.p.), pilocarpine (400 mg/kg, i.p.), picrotoxin (PTX) (7 mg/kg, i.p.), or strychnine (75 mg/kg, i.p.) induced seizure models. In the pilocarpine-induced seizure model, all dosages of agomelatine or melatonin showed a significant decrease in TBARS levels and nitrite content in all brain areas when compared to controls. In the strychnine-induced seizure model, all dosages of agomelatine and melatonin decreased TBARS levels in all brain areas, and agomelatine at low doses (25 or 50 mg/kg) and melatonin decreased nitrite contents, but only agomelatine at 25 or 50 mg/kg showed a significant increase in catalase activity in three brain areas when compared to controls. Neither melatonin nor agomelatine at any dose have shown no antioxidant effects on parameters of oxidative stress produced by PTX- or PTZ-induced seizure models when compared to controls. Our results suggest that agomelatine has antioxidant activity as shown in strychnine- or pilocarpine-induced seizure models.[3] - Dual Mechanism: Agomelatine acts as a melatonin receptor agonist to regulate circadian rhythms and a 5-HT2C antagonist to enhance monoaminergic neurotransmission [2] - Indications: Approved for major depressive disorder, with efficacy comparable to SSRIs but faster onset of action - Liver Monitoring: EMA recommends baseline and periodic liver function tests due to risk of hepatotoxicity |
| 分子式 |
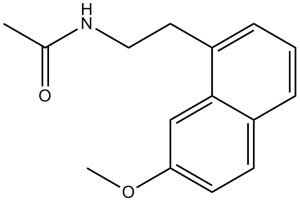
C15H17NO2
|
|---|---|
| 分子量 |
243.3
|
| 精确质量 |
243.125
|
| 元素分析 |
C, 74.05; H, 7.04; N, 5.76; O, 13.15
|
| CAS号 |
138112-76-2
|
| 相关CAS号 |
Agomelatine hydrochloride; 1176316-99-6; Agomelatine (L(+)-Tartaric acid); 824393-18-2; Agomelatine-d6; 1079389-42-6; Agomelatin-d3; 1079389-38-0; Agomelatine-d4; 1079389-44-8
|
| PubChem CID |
82148
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
478.8±28.0 °C at 760 mmHg
|
| 熔点 |
107-109ºC
|
| 闪点 |
243.4±24.0 °C
|
| 蒸汽压 |
0.0±1.2 mmHg at 25°C
|
| 折射率 |
1.582
|
| LogP |
2.27
|
| tPSA |
38.33
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
2
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
18
|
| 分子复杂度/Complexity |
280
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CC(NCCC1=C2C=C(OC)C=CC2=CC=C1)=O
|
| InChi Key |
YJYPHIXNFHFHND-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C15H17NO2/c1-11(17)16-9-8-13-5-3-4-12-6-7-14(18-2)10-15(12)13/h3-7,10H,8-9H2,1-2H3,(H,16,17)
|
| 化学名 |
N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide
|
| 别名 |
S20098; Valdoxan; Thymanax; Melitor; AGO 178; N-(2-(7-methoxy-1-naphthyl)ethyl)acetamide; S 20098; AGO-178; N-(2-(7-Methoxynaphthalen-1-yl)ethyl)acetamide; N-[2-(7-methoxynaphthalen-1-yl)ethyl]acetamide; AGO178; S-20098
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (10.28 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (10.28 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (10.28 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 4.1102 mL | 20.5508 mL | 41.1015 mL | |
| 5 mM | 0.8220 mL | 4.1102 mL | 8.2203 mL | |
| 10 mM | 0.4110 mL | 2.0551 mL | 4.1102 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT05426304 | Not yet recruiting | Drug: Agomelatine Drug: Placebo Tablets |
Depression Acute Ischemic Stroke |
First Affiliated Hospital, Sun Yat-Sen University |
October 1, 2022 | Phase 4 |
| NCT01822418 | Completed | Drug: agomelatine | Schizophrenia Delusional Disorder |
Central Institute of Mental Health, Mannheim |
January 2013 | Phase 4 |
| NCT01531309 | Completed | Drug: AGO178 | Hepatic Impairment | Novartis Pharmaceuticals | February 8, 2011 | Phase 1 |
| NCT01110902 | Completed | Drug: Placebo Drug: Agomelatine (AGO178C) |
Major Depressive Disorder | Novartis Pharmaceuticals | May 2010 | Phase 3 |
| NCT00411099 | Completed | Drug: agomelatine Drug: placebo |
Major Depressive Disorder | Novartis | December 2006 | Phase 3 |