| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| Other Sizes |
|

| 靶点 |
Daunorubicins/Doxorubicins; Topoisomerase II
|
|---|---|
| 体外研究 (In Vitro) |
Aldoxorubicin (INNO-206)(0.27 至 2.16 μM)以 pH 依赖性方式抑制血管形成并减少多发性骨髓瘤细胞的生长[1]。
|
| 体内研究 (In Vivo) |
Aldoxorubicin (INNO-206) (10.8 mg/kg, iv) 耐受性良好; 90% 患有 LAGκ-1A 肿瘤的小鼠能够存活到研究结论[1]。在第 28 天,它还表现出明显较小的肿瘤体积和 IgG 水平。 I 期研究表明,醛多柔比星 (INNO-206) 可以导致肉瘤、小细胞肺癌和乳腺癌的肿瘤消退,同时在剂量增加时保持良好的安全性至 260 mg/mL 阿霉素当量[2]。在乳腺癌异种移植物和鼠肾细胞癌模型中,醛柔比星(INNO-206)比阿霉素表现出更好的疗效[3]。
|
| 细胞实验 |
细胞活力测定[1]
在含有FBS的RPMI-1640培养基中,以1×105个细胞/100μL/孔的密度将细胞接种在96孔板上24小时,然后进行处理。细胞在培养基、INNO-206或阿霉素存在下培养48小时。接下来,使用CellTiter 96 AQueous非放射性细胞增殖试验(Promega)定量细胞存活率。每个孔用MTS处理1至4小时,之后使用96孔板读数器记录490nm处的吸光度。甲赞产物的测量量与活细胞的数量成正比。绘制的数据为平均值±SEM,每个数据点使用3个重复。 通过MTS法测定不同pH水平的INNO-206对多发性骨髓瘤细胞系增殖的抗多发性髓瘤作用,并通过绒毛尿囊膜/羽毛芽法测定其抗血管生成活性。使用我们的多发性骨髓瘤异种移植物模型,还将INNO-206的抗多发性髓瘤作用和毒性与常规阿霉素和聚乙二醇化脂质体阿霉素(PLD)单独使用以及与硼替佐米联合使用进行了比较[1]。 |
| 动物实验 |
INNO-206 stock solutions (5.4 mg/mL) were prepared using 50% ethanol and 50% water and further diluted in sterile water. [1]
For the LAGκ-1A experiment, INNO-206 was administered to SCID mice at 10.8 mg/kg (doxorubicin equivalent dose of 8.0 mg/kg) once weekly. Mice were treated with conventional doxorubicin at 4.0 and 8.0 mg/kg once weekly. For the LAGκ-2 experiment, INNO-206 was administered once weekly (W) at doses of 2.7 and 5.4 mg/kg, or on 3 consecutive days (W-F) weekly at doses of 0.9 and 1.8 mg/kg. Bortezomib was administered twice weekly (W, F) at a dose of 0.5 mg/kg. Doxorubicin was administered to SCID mice at 2, 4, and 8 mg/kg, and PLD was administered to SCID mice at 2 mg/kg once weekly. Each drug was administered i.v. in a volume of 100 μL.[1] The (6-maleimidocaproyl)hydrazone derivative of doxorubicin (INNO-206) is an albumin-binding prodrug of doxorubicin with acid-sensitive properties that is being assessed clinically. The prodrug binds rapidly to circulating serum albumin and releases doxorubicin selectively at the tumor site. This novel mechanism may provide enhanced antitumor activity of doxorubicin while improving the overall toxicity profile. Preclinically, INNO-206 has shown superior activity over doxorubicin in a murine renal cell carcinoma model and in breast carcinoma xenograft models. In this work, we compared the antitumor activity of INNO-206 and doxorubicin at their respective maximum tolerated doses in three additional xenograft models (breast carcinoma 3366, ovarian carcinoma A2780, and small cell lung cancer H209) as well as in an orthotopic pancreas carcinoma model (AsPC-1). INNO-206 showed more potent antitumor efficacy than free doxorubicin in all tumor models and is thus a promising clinical candidate for treating a broad range of solid tumors[3]. |
| 参考文献 |
|
| 其他信息 |
Aldoxorubicin, an antineoplastic agents, is an albumin-binding prodrug of doxorubicin.
Aldoxorubicin is a 6-maleimidocaproyl hydrazone derivative prodrug of the anthracycline antibiotic doxorubicin (DOXO-EMCH) with antineoplastic activity. Following intravenous administration, aldoxorubicin binds selectively to the cysteine-34 position of albumin via its maleimide moiety. Doxorubicin is released from the albumin carrier after cleavage of the acid-sensitive hydrazone linker within the acidic environment of tumors and, once located intracellularly, intercalates DNA, inhibits DNA synthesis, and induces apoptosis. Albumin tends to accumulate in solid tumors as a result of high metabolic turnover, rapid angiogenesis, hypervasculature, and impaired lymphatic drainage. Because of passive accumulation within tumors, this agent may improve the therapeutic effects of doxorubicin while minimizing systemic toxicity. Drug Indication Investigated for use/treatment in solid tumors. Mechanism of Action INNO-206 is the (6-Maleimidocaproyl) hydrazone of doxorubicin. INNO-206 is a prodrug of doxorubicin that binds endogenous albumin after administration. The bound doxorubicin is released in the acidic environment of the tumor cell through cleavage of an acid sensitive linker. In preclinical models, INNO-206 was superior to doxorubicin with regard to antitumor efficacy and toxicity. |
| 分子式 |
C37H42N4O13
|
|---|---|
| 分子量 |
750.748
|
| 精确质量 |
750.274
|
| 元素分析 |
C, 59.19; H, 5.64; N, 7.46; O, 27.70
|
| CAS号 |
1361644-26-9
|
| 相关CAS号 |
MC-DOXHZN hydrochloride;480998-12-7;MC-DOXHZN;151038-96-9;Aldoxorubicin hydrochloride;1361563-03-2
|
| PubChem CID |
9810709
|
| 外观&性状 |
Purple to purplish red solid powder
|
| 密度 |
1.6±0.1 g/cm3
|
| 折射率 |
1.708
|
| LogP |
3.08
|
| tPSA |
271.33
|
| 氢键供体(HBD)数目 |
7
|
| 氢键受体(HBA)数目 |
15
|
| 可旋转键数目(RBC) |
12
|
| 重原子数目 |
54
|
| 分子复杂度/Complexity |
1510
|
| 定义原子立体中心数目 |
6
|
| SMILES |
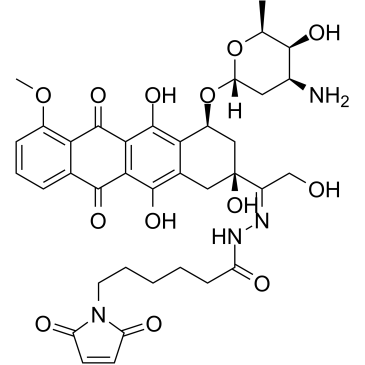
[H][C@@]1(O[C@H]2C[C@@](/C(CO)=N/NC(CCCCCN3C(C=CC3=O)=O)=O)(O)CC(C2=C4O)=C(O)C5=C4C(C6=C(OC)C=CC=C6C5=O)=O)O[C@@H](C)[C@@H](O)[C@@H](N)C1
|
| InChi Key |
OBMJQRLIQQTJLR-USGQOSEYSA-N
|
| InChi Code |
InChI=1S/C37H42N4O13/c1-17-32(46)20(38)13-27(53-17)54-22-15-37(51,23(16-42)39-40-24(43)9-4-3-5-12-41-25(44)10-11-26(41)45)14-19-29(22)36(50)31-30(34(19)48)33(47)18-7-6-8-21(52-2)28(18)35(31)49/h6-8,10-11,17,20,22,27,32,42,46,48,50-51H,3-5,9,12-16,38H2,1-2H3,(H,40,43)/b39-23+/t17-,20-,22-,27-,32+,37-/m0/s1
|
| 化学名 |
N-[(E)-[1-[(2S,4S)-4-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]-2-hydroxyethylidene]amino]-6-(2,5-dioxopyrrol-1-yl)hexanamide
|
| 别名 |
EMCH-doxorubicin; MC-DOXHZN hydrochloride; INNO 206; INNO-206 HCl; DOXO-EMCH; EMCH-Doxo; INNO206; INNO-206; Doxorubicin-EMCH; 151038-96-9; MC-Doxhzn; N-[(E)-[1-[(2S,4S)-4-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]-2-hydroxyethylidene]amino]-6-(2,5-dioxopyrrol-1-yl)hexanamide; Aldoxorubicin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: (1). 请将本产品存放在密封且受保护的环境中(例如氮气保护),避免吸湿/受潮。 (2). 该产品在溶液状态不稳定,请现配现用。 |
| 运输条件 |
Ship with dry ice
|
| 溶解度 (体外实验) |
DMSO: ≥ 50 mg/mL (~66.6 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (3.33 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (3.33 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (3.33 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.3320 mL | 6.6600 mL | 13.3200 mL | |
| 5 mM | 0.2664 mL | 1.3320 mL | 2.6640 mL | |
| 10 mM | 0.1332 mL | 0.6660 mL | 1.3320 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03387085 | Active Recruiting |
Drug: Aldoxorubicin HCl Biological: N-803 |
Triple Negative Breast Cancer | ImmunityBio, Inc. | March 16, 2018 | Phase 1 Phase 2 |
| NCT03563157 | Active Recruiting |
Biological: ALT-803 Biological: ETBX-011 |
Colorectal Cancer Metastatic mCRC |
ImmunityBio, Inc. | May 25, 2018 | Phase 1 Phase 2 |
| NCT04390399 | Recruiting | Drug: Aldoxorubicin HCl Biological: PD-L1 t-haNK |
Pancreatic Cancer | ImmunityBio, Inc. | July 21, 2020 | Phase 2 |
| NCT02029430 | Completed | Drug: aldoxorubicin | AIDS HIV Positive |
ImmunityBio, Inc. | January 2014 | Phase 2 |
| NCT02014844 | Completed | Drug: aldoxorubicin | Glioblastoma | ImmunityBio, Inc. | March 2014 | Phase 2 |
|
|
|
|
|