| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|

| 体内研究 (In Vivo) |
在缺乏钠的狨猴中,口服阿利吉仑(每日< 10 mg/kg)可降低血压并抑制血浆肾素活性[2]。在表达 C26 小鼠结肠癌细胞的 BALB/c 小鼠中,阿利吉仑(10 mg/kg,灌胃一次)可显着减轻许多恶病质相关症状,如体重减轻、肿瘤负荷、肌肉萎缩和肌肉功能障碍。并缩短寿命[3]。
|
|---|---|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Aliskiren is absorbed in the gastrointestinal tract and is poorly absorbed with a bioavailability between 2.0 and 2.5%. Peak plasma concentrations of aliskiren are achieved between 1 to 3 hours after administration. Steady-state concentrations of aliskiren are achieved within 7-8 days of regular administration. Aliskiren is mainly excreted via the hepatobiliary route and by oxidative metabolism by hepatic cytochrome enzymes. Approximately one-quarter of the absorbed dose appears in the urine as unchanged parent drug. One pharmacokinetic study of radiolabeled aliskiren detected 0.6% radioactivity in the urine and more than 80% in the feces, suggesting that aliskiren is mainly eliminated by the fecal route. Unchanged aliskiren accounts for about 80% of the drug found in the plasma. Aliskiren is partially cleared in the kidneys, and safety data have not been established for patients with a creatinine clearance of less than 30 mL/min. One pharmacokinetic study revealed an average renal clearance of 1280 +/- 500 mL/hour in healthy volunteers. Poorly absorbed; oral bioavailability is about 2.5%. Steady state blood levels are reached in about 7-8 days. Peak plasma concentrations usually attained within 1-3 hours following oral administration. Substantial proportion (85-90%) of antihypertensive effect attained within 2 weeks of initiation of therapy. High-fat meal decreases mean AUC and peak plasma concentration by 71 and 85%, respectively; however, in clinical studies drug was administered without requiring a fixed relation of administration to meals. For more Absorption, Distribution and Excretion (Complete) data for Aliskiren (12 total), please visit the HSDB record page. Metabolism / Metabolites About 80% of the drug in plasma following oral administration is unchanged. Two major metabolites account for about 1-3% of aliskiren in the plasma. One metabolite is an O-demethylated alcohol derivative and the other is a carboxylic acid derivative. Minor oxidized and hydrolyzed metabolites may also be found in the plasma. Amount of absorbed dose that undergoes metabolism not established; however, drug appears to undergo minimal hepatic metabolism. CYP isoenzyme 3A4 appears to be main enzyme responsible for metabolism of drug based on in vitro studies. Also, a substrate for p-glycoprotein. The major metabolic pathways for aliskiren were O-demethylation at the phenyl-propoxy side chain or 3-methoxy-propoxy group, with further oxidation to the carboxylic acid derivative. ... The two major oxidized metabolites of aliskiren account for less than 5% of the drug in the plasma at the time of the maximum concentration. ... Biological Half-Life Plasma half-life for aliskiren can range from 30 to 40 hours with an accumulation half-life of about 24 hours. Accumulation half-life is approximately 24 hours. ... Terminal half-lives for radioactivity and aliskiren in plasma were 49 hr and 44 hr, respectively. ... Terminal half-life is approximately 24-40 hours; wide interpatient variability observed. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Serum aminotransferase elevations during aliskiren therapy are uncommon and rates of such elevations have not been reported in the large clinical trials that demonstrated its efficacy in hypertension. An instance of serum aminotransferase elevation with jaundice was reported in the registration trials of aliskiren and several instances of marked serum enzyme elevations during therapy with few symptoms and without jaundice have been reported. In most instances, the pattern of enzyme elevation was distinctly hepatocellular and recovery was prompt once aliskiren was stopped. There have also been reports to the sponsor of severe hepatic reactions including hepatic failure and more recently a published case report of acute liver injury attributed to this agent. The latency to onset has ranged from 1 to 6 months and the pattern of injuryis typically cholestatic or mixed. Most cases have been mild to moderate in severity and marked by rapid recovery once aliskiren is stopped. Likelihood score: C (probable cause of clinically apparent liver injury). Protein Binding The plasma protein binding of aliskiren ranges from 47-51%. Interactions Furosemide: When aliskiren was coadministered with furosemide, the AUC and Cmax of furosemide were reduced by about 30% and 50%, respectively. Patients receiving furosemide could find its effect diminished after starting aliskiren. Verapamil: Coadministration of 240 mg of verapamil with 300 mg aliskiren resulted in an approximately 2-fold increase in Cmax and AUC of aliskiren. However, no dosage adjustment is necessary. Cyclosporine: Coadministration of 200 mg and 600 mg cyclosporine with 75 mg aliskiren resulted in an approximately 2.5-fold increase in Cmax and 5-fold increase in AUC of aliskiren. Concomitant use of aliskiren with cyclosporine is not recommended. Ketoconazole: Coadministration of 200 mg twice-daily ketoconazole with aliskiren resulted in an approximate 80% increase in plasma levels of aliskiren. A 400-mg once-daily dose was not studied but would be expected to increase aliskiren blood levels further. For more Interactions (Complete) data for Aliskiren (11 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antihypertensive Agent; Renin/antagonists and inhibitors Tekturna is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents. Use with maximal doses of ACE inhibitors has not been adequately studied. /Included in US product label/ Drug Warnings /BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Tekturna as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. Possible fetal and neonatal morbidity and mortality when used during pregnancy. Such potential risks occur throughout pregnancy, especially during the second and third trimesters. Retrospective data indicate that angiotensin-converting enzyme (ACE) inhibitors, a class of drugs acting on the renin-angiotensin-aldosterone (RAA) system, have been associated with an increased risk of major congenital malformations when administered during the first trimester of pregnancy. Excessive hypotension reported rarely in patients with uncomplicated hypertension receiving the drug alone and infrequently during combination therapy with other antihypertensive agents. For more Drug Warnings (Complete) data for Aliskiren (21 total), please visit the HSDB record page. Pharmacodynamics Aliskiren reduces blood pressure by inhibiting renin. This leads to a cascade of events that decreases blood pressure, lowering the risk of fatal and nonfatal cardiovascular events including stroke and myocardial infarction. |
| 分子式 |
C₃₀H₅₃N₃O₆
|
|---|---|
| 分子量 |
551.76
|
| 精确质量 |
551.393
|
| CAS号 |
173334-57-1
|
| 相关CAS号 |
Aliskiren hemifumarate;173334-58-2;Aliskiren hydrochloride;173399-03-6;Aliskiren-d6 hydrochloride;1246815-96-2;Aliskiren fumarate;1196835-68-3;Aliskiren-d6 hemifumarate
|
| PubChem CID |
5493444
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.1±0.1 g/cm3
|
| 沸点 |
748.4±60.0 °C at 760 mmHg
|
| 熔点 |
>95
|
| 闪点 |
406.4±32.9 °C
|
| 蒸汽压 |
0.0±2.6 mmHg at 25°C
|
| 折射率 |
1.514
|
| LogP |
2.74
|
| tPSA |
146.13
|
| 氢键供体(HBD)数目 |
4
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
19
|
| 重原子数目 |
39
|
| 分子复杂度/Complexity |
717
|
| 定义原子立体中心数目 |
4
|
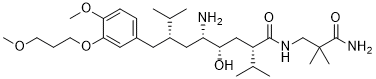
| SMILES |
CC(C)[C@@H](CC1=CC(=C(C=C1)OC)OCCCOC)C[C@@H]([C@H](C[C@@H](C(C)C)C(=O)NCC(C)(C)C(=O)N)O)N
|
| InChi Key |
UXOWGYHJODZGMF-QORCZRPOSA-N
|
| InChi Code |
InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1
|
| 化学名 |
(2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2-ylnonanamide
|
| 别名 |
CGP-60536B SPP100 CGP 60536 SPP-100CGP60536B SPP 100
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
Ethanol : ~100 mg/mL (~181.24 mM)
DMSO : ~100 mg/mL (~181.24 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.53 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.53 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.53 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.8124 mL | 9.0619 mL | 18.1238 mL | |
| 5 mM | 0.3625 mL | 1.8124 mL | 3.6248 mL | |
| 10 mM | 0.1812 mL | 0.9062 mL | 1.8124 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Effect of Aliskiren and Hydrochlorothiazide on Kidney Oxygenation in Patients With Hypertension
CTID: NCT01519635
Phase: Phase 4 Status: Completed
Date: 2020-03-17