| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
human OX2R ( Kd = 0.17 nM ); human OX1R ( Kd = 1.3 nM ); Caspase-3
|
||
|---|---|---|---|
| 体外研究 (In Vitro) |
体外活性:Almorexant 抑制中国仓鼠卵巢细胞中 10 nM 人食欲素-A 诱导的细胞内 Ca2+ 增加,对 OX1 受体的 IC50 分别为 16 nM(大鼠)和 13 nM(人),对 OX1 受体的 IC50 分别为 15 nM(大鼠)和 8 nM(人)代表 OX2 受体。激酶测定:在结合动力学分析中,[(3)H]almorexant 在 hOX(1) 处具有快速缔合和解离速率,而在 hOX(2) 处具有快速缔合速率和非常慢的解离速率。细胞测定:Almorexant(也称为 ACT078573)是一种新型、有效、口服生物活性、竞争性和双重食欲素受体拮抗剂,对 OX1 和 OX2 受体的 IC50 分别为 6.6 nM 和 3.4 nM。它具有治疗失眠的潜力。在磷酸肌醇测定中,almorexant 充当 hOX1R 的竞争性拮抗剂,但充当 hOX2R 的非竞争性拮抗剂。此外,almorexant 对包括人类在内的多个物种的睡眠都有影响。
|
||
| 体内研究 (In Vivo) |
Almorexant (300 mg/kg po) 降低雄性 Wistar 大鼠的警觉性,并增加非快速眼动睡眠和快速眼动睡眠的电生理指数。在狗中,Almorexant(100 mg/kg,口服)会导致嗜睡并增加快速眼动睡眠的替代标志物。 Almorexant 诱导强大的抗抑郁样作用,并恢复与压力相关的 HPA 轴缺陷,独立于神经源性作用。此外,Almorexant 还可以减少高饮酒啮齿动物模型中的乙醇自我给药。
|
||
| 酶活实验 |
最近的临床前和临床研究表明,Almorexant促进动物和人类的睡眠,而不破坏睡眠结构。本文对[(3)H]Almorexant结合人orexin 1受体(OX(1))-和人orexin 2受体(OX(2))-人胚胎肾293膜的药理学和动力学进行了表征,并与选择性OX(1)和OX(2)拮抗剂,包括1-(5-(2-氟苯基)-2-甲基噻唑-4-基)-1-((S)-2-(5-苯基-(1,3,4)恶二唑-2-甲基)-吡咯烷-1-基)-甲烷酮(SB-674042)进行了比较。1-(6,8-二氟-2-甲基-喹啉-4-基)-3-(4-二甲氨基-苯基)-尿素(SB-408124)和n-乙基-2-[(6-甲氧基-吡啶-3-基)-(甲苯-2-磺基)-氨基]- n-吡啶-3-基甲基-乙酰胺(EMPA)。体外实验还检测了这些拮抗剂对大鼠腹侧被盖区(VTA)多巴胺能神经元自发活动的影响。[(3)H]Almorexant结合到hOX(1)和hOX(2)上的单个饱和位点,具有高亲和力(K(d)分别为1.3 nM和0.17 nM)。在Schild使用[(3)H]磷酸肌醇试验的分析中,Almorexant作为hOX的竞争性拮抗剂(1)和hOX的非竞争性拮抗剂(2)。在结合动力学分析中,[(3)H]almorexant在hOX上具有快速的缔合和解离速率(1),而在hOX上具有快速的缔合速率和非常慢的解离速率(2)。在VTA中,食欲素- a在大约一半的测试神经元中增强了基础放电频率,达到对照的175 +/- 17%。在1微米SB-674042或SB-408124的存在下,食欲素- a的作用仅被部分拮抗。而在1 μ m EMPA或1 μ m Almorexant存在时,orexin-A的作用被完全拮抗。综上所述,Almorexant表现出一种非竞争性和持久的伪不可逆拮抗模式,因为它与OX的解离速度非常慢(2)。电生理数据表明,OX(2)可能比OX(1)更重要地介导食欲素- a对VTA多巴胺能神经元的慢放电作用[2]。
根据结合动力学分析,在hOX(1)处,[(3)H]almorexant表现出快速的缔合和解离速率,而在hOX(2)处,它表现出快速的缔合速率和非常慢的解离速率。 |
||
| 细胞实验 |
annexin V标记定量凋亡细胞[1]
按上述方法培养AsPC-1、SW 1990、hpf - ii和hpf - ii /hOX1R细胞(5 × 104个/孔)。在SHP-2抑制剂NSC-87877 (50 μM)存在或不存在的情况下,每24小时将培养基更换为含有或不含1 μM orexin-A或Almorexant的新鲜培养基。48小时后,使用Guava NexinTM试剂盒检测凋亡细胞。结果表示为凋亡的植物红蛋白标记的膜联蛋白V (Annexin V- pe)阳性细胞的百分比,是3个独立分析的结果。 Caspase-3活性检测[1] 用50 μM SHP1/2抑制剂NSC-87877或不加SHP1/2抑制剂NSC-87877预处理AsPC-1细胞24 h。5.106半流利细胞在新鲜培养基中用1 μM orexin-A或1 μM Almorexant在37°C下处理24 h。根据制造商的说明,使用Caspase-3测定比色试剂盒进行Caspase-3活性检测。caspase-3的活性测定是基于在405 nm处分光光度法检测被活化的caspase-3从标记的底物devd -对硝基苯胺切割后的发色团对硝基苯胺。结果表示为每个样品中200 μg蛋白质在405 nm处的光密度(od),是3次独立分析的平均值。 Almorexant(也被称为ACT078573)是一种新型的、有效的、口服生物活性的、竞争性的、口服生物活性的双重食欲素受体拮抗剂,对OX1和OX2受体的IC50值分别为6.6 nM和3.4 nM。它可以用来治疗失眠。Almorexant在肌醇磷酸试验中作为hOX1R的竞争性拮抗剂和hOX2R的非竞争性拮抗剂。此外,Almorexant影响包括人类在内的多种物种的睡眠。 |
||
| 动物实验 |
|
||
| 药代性质 (ADME/PK) |
The mean plasma concentration-time profiles and the corresponding pharmacokinetic variables of almorexant are shown in Figure 1 and Table 1, respectively. Under fasting conditions, almorexant was rapidly absorbed, with a median tmax of 1.5 hours at all doses. After maximum plasma concentration (Cmax) was attained, plasma almorexant concentrations decreased quickly by 80% to 90% over the 8 hours after the tmax. Whereas the terminal elimination half-life (t1/2) was 32 hours, the t1/2α associated with the distribution phase, which is responsible for the major part of the drug disposition from plasma, varied from 1.4 to 1.7 hours between the dose groups. In line with low concentrations 8 hours after the tmax, simulation of multiple dosing conditions indicated minimal accumulation. The pharmacokinetics of almorexant were dose proportional, with a value (95% confidence limits [CI]) for the dose proportionality coefficient, β, of 1.11 (0.68–1.55) for the Cmax and 1.16 (0.87–1.46) for area under the concentration-time curve (AUC)0-∞. Plasma concentrations of zolpidem reached a maximum within 2 hours in all subjects, and the median tmax was 0.92 hours. Subsequently, zolpidem concentrations quickly decreased, and the mean terminal t1/2 was 3.1 hours (Table 1).
Compared with healthy adult male subjects, the essential pharmacokinetic characteristics of almorexant as a possible sleep-enabling agent, that is, rapid absorption and low drug concentrations 8 hours after dosing, were preserved in healthy elderly subjects. Nevertheless, some differences can be noted: at a dose of 200 mg, mean values (elderly vs younger subjects) for Cmax (166 vs 134 ng/mL), AUC0-∞ (722 vs 430 ng·h/mL), and t1/2 (31.8 vs 14.4 h) were higher in the elderly subjects compared with the adult subjects. The t1/2α associated with the distribution phase, which is responsible for the major part of drug disposition from plasma, was approximately 1.6 hours. The observed longer t1/2 and consequently greater AUC0-∞ may be explained by the extended blood-sampling scheme (72 hours in this study vs 36 hours in the previous study in adult male subjects), which allowed for a better estimation of t1/2 in this study. In addition, an effect of age on the clearance of almorexant by CYP3A4 cannot be excluded. In both populations, the pharmacokinetics of almorexant were approximately dose proportional but variable with a coefficient of variation of approximately 50%. The pharmacokinetics of zolpidem in the elderly subjects showed a higher Cmax and AUC0-∞ and longer t1/2 compared with the adult subjects consistent with previous reports. Reference: https://pubmed.ncbi.nlm.nih.gov/23609389/ |
||
| 毒性/毒理 (Toxicokinetics/TK) |
No serious AEs were reported, and all AEs resolved without sequelae. As expected with a sleep-enabling compound, somnolence and fatigue were reported frequently. Other frequent AEs included headache and nausea. Four subjects with muscle weakness, 3 subjects on almorexant 400 mg and one subject on placebo were reported; among them, 3 were mentioned retrospectively during self-assessment using the narcoleptic effects questionnaire. The total number of different AEs reported was higher with the 400 mg than with the other almorexant doses. None of the AEs reported with placebo or almorexant were of severe intensity.
There were no clinically relevant effects of almorexant on vital signs, electrocardiogram, body weight, clinical laboratory variables, and physical examination.
Administration of single morning doses of almorexant to healthy elderly subjects was well tolerated, with no severe or serious AEs, and no observed effects on clinical laboratory variables, vital signs, body temperature, body weight, or quantitative ECG variables. It should be emphasized that frail elderly subjects did not take part in the study and that no subjects older than 81 years were enrolled. The tolerability profile of almorexant in this study in healthy elderly subjects is similar to the one reported for healthy male adults who had received a single morning dose of almorexant from 1 to 1000 mg. Any AEs related to possible muscle tone abnormalities, sleep paralysis, and hallucinations should be carefully monitored in future studies with orexin receptor antagonists as narcolepsy could be theoretically an adverse effect of orexin receptor antagonists given that orexin levels are decreased in patients with this disorder. Reference: https://pubmed.ncbi.nlm.nih.gov/23609389/ |
||
| 参考文献 |
|
||
| 其他信息 |
Almorexant is a member of isoquinolines.
Drug Indication Investigated for use/treatment in sleep disorders and insomnia. Pancreatic ductal adenocarcinoma (PDAC) is still the poorest prognostic tumor of the digestive system. We investigated the antitumoral role of orexin-A and almorexant in PDAC. We analyzed the orexin receptor type 1 (OX1R) expression by immunohistochemistry in human normal pancreas, PDAC and its precursor dysplastic intraepithelial lesions. We used PDAC-derived cell lines and fresh tissue slices to study the apoptotic role of hypocretin-1/orexin-A and almorexant in vitro and ex vivo. We analyzed in vivo the hypocretin-1/orexin-A and almorexant effect on tumor growth in mice xenografted with PDAC cell lines expressing, or not, OX1R. Ninety-six percent of PDAC expressed OX1R, while adjacent normal exocrine pancreas did not. OX1R was expressed in pre-cancerous lesions. In vitro, under hypocretin-1/orexin-A and almorexant, the OX1R-positive AsPC-1 cells underwent apoptosis, abolished by the tyrosine phosphatase SHP2 inhibitor, NSC-87877, whereas the OX1R-negative HPAF-II cell line did not. These effects were mediated by phosphorylation of OX1R and recruitment of SHP2. Ex vivo, caspase-3 positive tumor cells were significantly higher in fresh tumour slices treated 48h with hypocretin-1/orexin-A, as compared to control, whereas cellular proliferation, assessed by Ki-67 index, was not modified. In vivo, when AsPC-1 cells or patient-derived cells were xenografted in nude mice, hypocretin-1/orexin-A or almorexant, administrated both starting the day of cell line inoculation or after tumoral development, strongly slowed tumor growth. Hypocretin-1/orexin-A and almorexant induce, through OX1R, the inhibition of PDAC cellular growth by apoptosis. Hypocretins/orexins and almorexant might be powerful candidates for the treatment of PDAC.[1] Study objectives: Humans with narcolepsy and orexin/ataxin-3 transgenic (TG) mice exhibit extensive, but incomplete, degeneration of hypo-cretin (Hcrt) neurons. Partial Hcrt cell loss also occurs in Parkinson disease and other neurologic conditions. Whether Hcrt antagonists such as almorexant (ALM) can exert an effect on the Hcrt that remains after Hcrt neurodegeneration has not yet been determined. The current study was designed to evaluate the hypnotic and cataplexy-inducing efficacy of a Hcrt antagonist in an animal model with low Hcrt tone and compare the ALM efficacy profile in the disease model to that produced in wild-type (WT) control animals. Design: Counterbalanced crossover study. Setting: Home cage. Patients or participants: Nine TG mice and 10 WT mice. Interventions: ALM (30, 100, 300 mg/kg), vehicle and positive control injections, dark/active phase onset. [3] Rationale: Orexins play a key role in the maintenance of alertness and are implicated in the modulation of diverse physiological processes, including cognitive function. Almorexant, a dual orexin receptor antagonist, transiently and reversibly blocks the action of orexin peptides at both OX(1) and OX(2) receptors and increases time spent in rapid eye movement (REM) and non-REM sleep. Objectives: We explored the direct effects on learning and memory of single and repeated administration of almorexant in rats. Methods: Following administration of high doses of almorexant (300 mg/kg, p.o.), scopolamine (0.8 mg/kg, i.p.), combination almorexant-scopolamine, or vehicle alone, rats were trained on a Morris water maze spatial navigation task, or on a passive avoidance task. Results: Rats treated with almorexant learned the spatial navigation task with similar efficacy as vehicle-treated animals. After 4 days, almorexant-but not vehicle-treated rats had established spatial memory; after 8 days, spatial memory had been established in both vehicle-and almorexant-treated rats. Scopolamine-treated rats failed to learn the spatial task. Both vehicle-and almorexant-but not scopolamine-treated rats demonstrated passive avoidance learning. Almorexant did not ameliorate scopolamine-induced impairment of learning in either task. Conclusions: Rats treated with almorexant are fully capable of spatial and avoidance learning.[4] BPH/2J mice are a genetic model of hypertension associated with an overactive sympathetic nervous system. Orexin is a neuropeptide which influences sympathetic activity and blood pressure. Orexin precursor mRNA expression is greater in hypothalamic tissue of BPH/2J compared with normotensive BPN/3J mice. To determine whether enhanced orexinergic signaling contributes to the hypertension, BPH/2J and BPN/3J mice were preimplanted with radiotelemetry probes to compare blood pressure 1 hour before and 5 hours after administration of almorexant, an orexin receptor antagonist. Mid frequency mean arterial pressure power and the depressor response to ganglion blockade were also used as indicators of sympathetic nervous system activity. Administration of almorexant at 100 (IP) and 300 mg/kg (oral) in BPH/2J mice during the dark-active period (2 hours after lights off) markedly reduced blood pressure (-16.1 ± 1.6 and -11.0 ± 1.1 mm Hg, respectively;P<0.001 compared with vehicle). However, when almorexant (100 mg/kg, IP) was administered during the light-inactive period (5 hours before lights off) no reduction from baseline was observed (P=0.64). The same dose of almorexant in BPN/3J mice had no effect on blood pressure during the dark (P=0.79) or light periods (P=0.24). Almorexant attenuated the depressor response to ganglion blockade (P=0.018) and reduced the mid frequency mean arterial pressure power in BPH/2J mice (P<0.001), but not BPN/3J mice (P=0.70). Immunohistochemical labeling revealed that BPH/2J mice have 29% more orexin neurons than BPN/3J mice which are preferentially located in the lateral hypothalamus. The results suggest that enhanced orexinergic signaling contributes to sympathetic overactivity and hypertension during the dark period in BPH/2J mice.[5] |
| 分子式 |
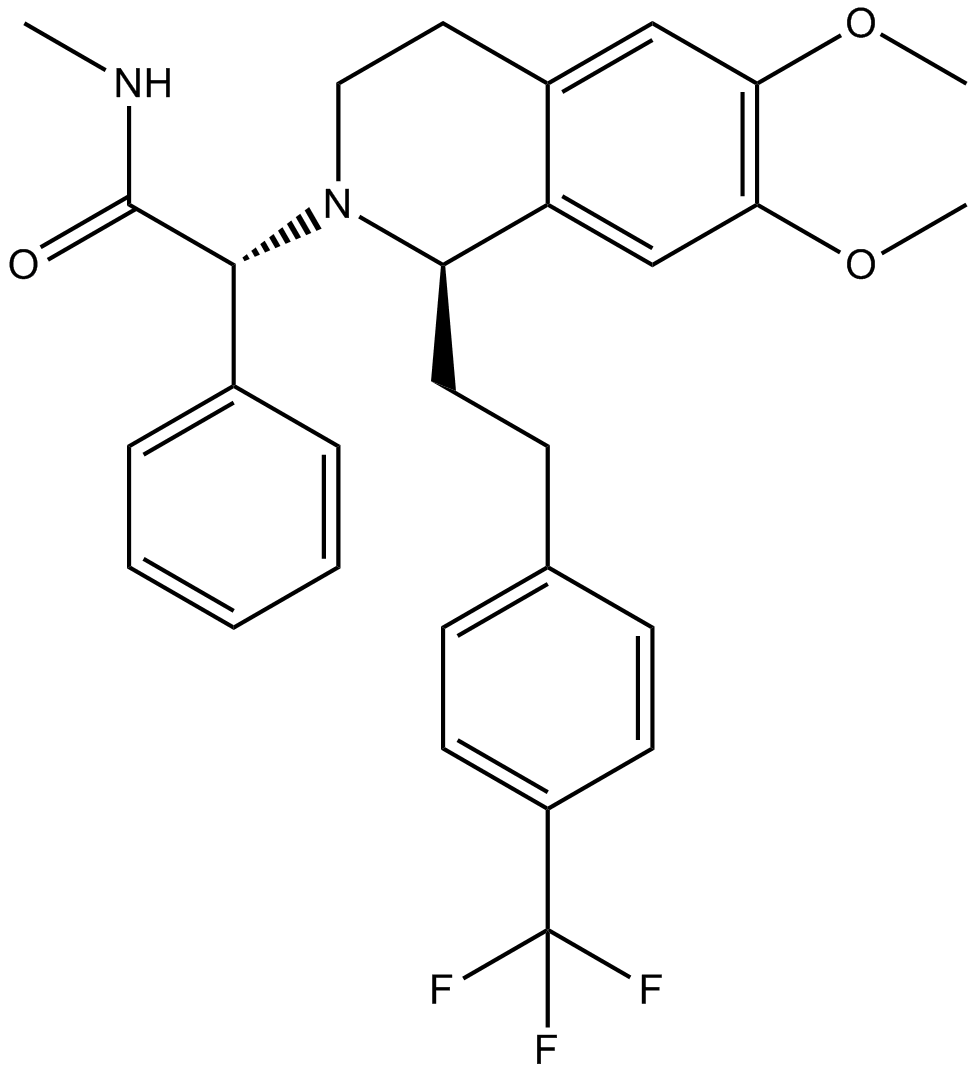
C29H31F3N2O3
|
|
|---|---|---|
| 分子量 |
512.56
|
|
| 精确质量 |
512.228
|
|
| 元素分析 |
C, 67.95; H, 6.10; F, 11.12; N, 5.47; O, 9.36
|
|
| CAS号 |
871224-64-5
|
|
| 相关CAS号 |
Almorexant hydrochloride; 913358-93-7; Almorexant-13C,d3; 871224-64-5
|
|
| PubChem CID |
23727689
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
620.4±55.0 °C at 760 mmHg
|
|
| 闪点 |
329.0±31.5 °C
|
|
| 蒸汽压 |
0.0±1.8 mmHg at 25°C
|
|
| 折射率 |
1.554
|
|
| LogP |
5.89
|
|
| tPSA |
50.8
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
7
|
|
| 可旋转键数目(RBC) |
8
|
|
| 重原子数目 |
37
|
|
| 分子复杂度/Complexity |
722
|
|
| 定义原子立体中心数目 |
2
|
|
| SMILES |
C([C@@H]1N([C@H](C2C=CC=CC=2)C(=O)NC)CCC2=CC(=C(C=C12)OC)OC)CC1C=CC(C(F)(F)F)=CC=1
|
|
| InChi Key |
DKMACHNQISHMDN-RPLLCQBOSA-N
|
|
| InChi Code |
InChI=1S/C29H31F3N2O3/c1-33-28(35)27(20-7-5-4-6-8-20)34-16-15-21-17-25(36-2)26(37-3)18-23(21)24(34)14-11-19-9-12-22(13-10-19)29(30,31)32/h4-10,12-13,17-18,24,27H,11,14-16H2,1-3H3,(H,33,35)/t24-,27+/m0/s1
|
|
| 化学名 |
(2R)-2-[(1S)-6,7-dimethoxy-1-[2-[4-(trifluoromethyl)phenyl]ethyl]-3,4-dihydro-1H-isoquinolin-2-yl]-N-methyl-2-phenylacetamide
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.88 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9510 mL | 9.7550 mL | 19.5099 mL | |
| 5 mM | 0.3902 mL | 1.9510 mL | 3.9020 mL | |
| 10 mM | 0.1951 mL | 0.9755 mL | 1.9510 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT00608985 | Completed | Drug: almorexant Drug: Placebo |
Primary Insomnia | Midnight Pharma, LLC | March 2008 | Phase 3 |
| NCT01243060 | Completed | Drug: Almorexant Drug: Zolpidem 10mg |
Healthy Volunteers | Northern California Institute of Research and Education | May 2011 | Not Applicable |
| NCT00640848 | Completed | Drug: almorexant | Schizoaffective Disorder Schizophrenia |
Insomnia Primary Insomnia |
May 2006 | Phase 1 |
| NCT01987739 | Completed | Drug: 200 mg almorexant Drug: 400 mg almorexant |
Abuse Potential Study | Midnight Pharma, LLC | September 2009 | Phase 1 |
  |
 |