| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
p53 activator; TrxR1 inhibitor
Eprenetapopt (APR246; PRIMA-1MET) targets mutant p53 (mutp53) proteins, reactivating their transcriptional activity with EC50 values ranging from 2.5–10 μM in mutp53-bearing cancer cell lines[2] Eprenetapopt (APR246; PRIMA-1MET) indirectly induces reactive oxygen species (ROS) production, with no direct binding to specific enzymatic targets[1] |
|---|---|
| 体外研究 (In Vitro) |
APR-246 (PRIMA-1MET) 是第一个临床阶段的化合物,可重新激活突变型 p53 并诱导细胞凋亡。 APR-246 是一种前药,可转化为活性成分亚甲基奎宁环酮 (MQ),这是一种迈克尔受体,可与突变体 p53 中的半胱氨酸残基结合并将其恢复到野生型构象。 APR-246 可完全恢复具有 p53 突变的耐药卵巢癌细胞对顺铂和阿霉素的敏感性。除了重新激活 p53 之外,它还可以剂量依赖性地降低细胞内谷胱甘肽水平。通过增加 ROS 和 ER 应激、抑制硫氧还蛋白还原酶 1 (TrxR1) 以及诱导 ROS 和 ER 应激,APR-246 可以以不依赖于 p53 的方式引起细胞凋亡。此外,值得注意的是,APR-246 会改变骨髓瘤细胞中的 GSH/ROS 平衡,导致细胞死亡,无论 p53 状态如何[1]。在体外,PRIMA-1Met/APR-246 有效地阻止了表达突变型 p53 的 SCLC 细胞系的生长。它还会引起细胞凋亡,其特点是细胞内DNA片段化比例增加、caspase-3激活、PARP裂解、Bax和Noxa上调、Bcl-2下调[2]。
表达mutp53的癌细胞系(SKOV3、OVCAR3、A2780/CP70)中,依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(5–20 μM)孵育72小时后,剂量依赖性抑制细胞增殖40–70%,EC50值分别为4.2 μM(SKOV3)、5.8 μM(OVCAR3)和7.5 μM(A2780/CP70)[2] - 该化合物(10 μM)重激活mutp53,在SKOV3细胞中上调下游靶基因mRNA和蛋白表达:p21升高2.5倍,Bax升高2.0倍,PUMA升高1.8倍[2] - 转染mutp53(R175H、R273H)的HCT116 p53⁻/⁻细胞中,依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(15 μM)诱导60–70%的细胞凋亡,表现为膜联蛋白V/PI染色阳性,半胱天冬酶-3、-7及多聚ADP核糖聚合酶(PARP)出现剪切条带[1] - 依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(5–20 μM)使mutp53癌细胞内ROS水平升高1.5–3.0倍,ROS清除剂(NAC)可阻断其凋亡诱导作用[1] - 与顺铂(1 μM)联合使用时,依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(5 μM)协同抑制OVCAR3细胞增殖(抑制率从35%提升至65%),并增强顺铂诱导的凋亡[2] |
| 体内研究 (In Vivo) |
APR-246 在前列腺癌和血液恶性肿瘤的 I/II 期临床剂量探索研究中表现出良好的安全性,并且注意到临床和 p53 依赖性生物反应。在动物研究中,APR-246 具有良好的耐受性。当具有快速生长的 A2780-CP20 肿瘤异种移植物的小鼠仅给予一剂 APR-246 时,肿瘤大小减小了 21%[1]。
SKOV3(mutp53)异种移植裸鼠模型:依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET) 以100 mg/kg剂量每周腹腔注射2次,连续4周,与溶媒对照组相比,肿瘤体积缩小60%,肿瘤重量减轻55%[2] - 肿瘤组织免疫组织化学分析显示,mutp53核定位增加,p21和Bax表达上调,TUNEL阳性凋亡细胞比例增加45%[2] - HCT116 p53⁻/⁻(转染R175H mutp53)异种移植小鼠模型:依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET) 以150 mg/kg剂量每日口服给药3周,中位生存期延长30%,肿瘤生长抑制50%[1] - 治疗组小鼠未出现明显体重下降或器官毒性,血清谷丙转氨酶(ALT)、谷草转氨酶(AST)和肌酐水平均在正常范围内[1,2] |
| 酶活实验 |
将细胞以每平方厘米 15 000 个细胞的密度接种在六孔板中。第二天,用不同浓度的 APR-246(0、25、50、75 和 100 μM)处理细胞,并在 4、12 和 24 小时后收获。裂解细胞,澄清的上清液用于 TrxR 酶活性分析或蛋白质印迹分析。总蛋白浓度用 Bradford 试剂盒测定。使用针对微孔板的适应性 Trx 依赖性终点胰岛素减少测定来测量细胞 TrxR 活性。
mutp53转录活性实验:表达mutp53的癌细胞(SKOV3)接种于24孔板,转染p53响应性荧光素酶报告质粒。24小时后加入依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(2.5–20 μM),继续培养24小时。 luminometer检测荧光素酶活性,计算相对于溶媒对照组的相对转录活性[2] - ROS检测实验:mutp53癌细胞(HCT116 R175H)用DCFH-DA探针(10 μM)负载30分钟,再用依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(5–20 μM)处理24小时。流式细胞仪在激发波长488 nm、发射波长525 nm下检测荧光强度(反映ROS水平)[1] |
| 细胞实验 |
在 12 孔板中,每孔铺有 3 ml 培养基和 75 000 个 OVCAR-3 细胞。第二天除去2.5ml培养基后,用顺铂、APR-246或两者处理细胞20小时。第二天,通过胰蛋白酶消化收集细胞,洗涤两次,并用膜联蛋白 V 和碘化丙啶 (PI) 染色。对样品进行染色,然后使用LSRII流式细胞仪进行分析。
细胞增殖实验:表达mutp53的癌细胞(SKOV3、OVCAR3、A2780/CP70)以每孔5×10³个细胞接种于96孔板,过夜孵育。加入依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(1–40 μM),培养72小时。MTT法检测细胞活力,GraphPad Prism软件计算EC50值[2] - 凋亡实验:转染mutp53(R175H、R273H)的HCT116 p53⁻/⁻细胞接种于6孔板,用依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(15 μM)处理24小时。细胞用膜联蛋白V-FITC和PI染色,流式细胞仪分析;Western blot检测剪切型caspase-3、-7及PARP[1] - p53靶基因Western blot实验:SKOV3细胞用依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(10 μM)处理24小时后裂解,蛋白质经SDS-PAGE分离,转膜后与mutp53、p21、Bax、PUMA及β-肌动蛋白抗体孵育,化学发光法检测信号[2] - 联合治疗实验:OVCAR3细胞分别用依普奈特凋亡素(Eprenetapopt, APR246; PRIMA-1MET)(5 μM)单药、顺铂(1 μM)单药或两者联合处理72小时。MTT法检测细胞活力,通过联合指数(CI < 1)评估协同作用[2] |
| 动物实验 |
Dissolved in PBS; 400 mg/kg/day; i.v. injection
CD-1 Nu/Nu mice SKOV3 xenograft model: Female nude mice (6–8 weeks old) were subcutaneously inoculated with 5×10⁶ SKOV3 cells in Matrigel (1:1). When tumors reached 100–150 mm³, mice were randomly divided into vehicle and treatment groups (n=6/group). Eprenetapopt (APR246; PRIMA-1MET) was dissolved in 5% DMSO + 95% saline and administered intraperitoneally at 100 mg/kg twice weekly for 4 weeks. Tumor volume was measured every 3 days, and mice were euthanized for tumor weight and immunohistochemical analysis[2] - HCT116 R175H xenograft model: Male nude mice (6–8 weeks old) were subcutaneously implanted with 2×10⁶ HCT116 p53⁻/⁻ cells transfected with mutp53 R175H. After tumor establishment (100 mm³), mice received Eprenetapopt (APR246; PRIMA-1MET) (150 mg/kg/day) dissolved in 0.5% CMC via oral gavage for 3 weeks. Survival was monitored daily, and tumor volume was measured twice weekly[1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In vitro, Eprenetapopt (APR246; PRIMA-1MET) showed low cytotoxicity to normal human fibroblasts (NHF) with CC50 > 40 μM, resulting in a therapeutic index (CC50/EC50) > 8 in SKOV3 cells[2]
- In vivo, repeated administration of Eprenetapopt (APR246; PRIMA-1MET) (100 mg/kg ip twice weekly or 150 mg/kg po daily) did not cause significant body weight loss (<5% change vs. control) or gross pathological abnormalities in liver, kidney, spleen, or heart[1,2] - Serum levels of ALT, AST, creatinine, and urea nitrogen in treated mice were comparable to vehicle control, indicating no hepatotoxicity or nephrotoxicity[1,2] |
| 参考文献 | |
| 其他信息 |
Eprenetapopt has been used in trials studying the treatment of Prostatic Neoplasms, Hematologic Neoplasms, and Platinum Sensitive Recurrent High-grade Serous Ovarian Cancer With Mutated p53.
Eprenetapopt is a methylated derivative and structural analog of PRIMA-1 (p53 re-activation and induction of massive apoptosis), with potential antineoplastic activity. Upon administration, eprenetapopt covalently modifies the core domain of mutated forms of cellular tumor antigen p53 (p53) through the alkylation of thiol groups. These modifications restore both the wild-type conformation and function to mutant p53, which reconstitutes endogenous p53 activity, leading to cell cycle arrest and apoptosis in tumor cells. This agent may work synergistically with other antineoplastic agents. p53, a tumor suppressor and transcription factor normally activated upon DNA damage, is frequently mutated and overexpressed in cancer cells; it plays a key role in both DNA repair and the induction of apoptosis. Eprenetapopt (APR246; PRIMA-1MET) is a small-molecule compound that reactivates mutant p53 proteins by restoring their proper folding and transcriptional activity[1,2] - Its anticancer mechanism involves two key pathways: reactivation of mutp53 to induce cell cycle arrest and apoptosis via upregulating p53 target genes (p21, Bax, PUMA), and induction of intracellular ROS accumulation to trigger apoptotic signaling[1] - The compound exhibits synergistic anticancer effects with chemotherapeutic agents (e.g., cisplatin) in mutp53-expressing cancer cells, enhancing treatment efficacy[2] - It is being developed for the treatment of solid tumors harboring p53 mutations, including ovarian cancer, colorectal cancer, and non-small cell lung cancer[1,2] |
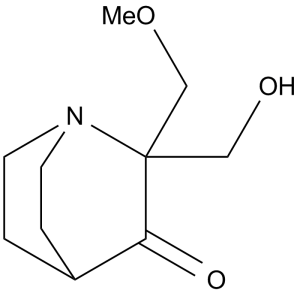
| 分子式 |
C10H17NO3
|
|
|---|---|---|
| 分子量 |
199.25
|
|
| 精确质量 |
199.12
|
|
| 元素分析 |
C, 60.28; H, 8.60; N, 7.03; O, 24.09
|
|
| CAS号 |
5291-32-7
|
|
| 相关CAS号 |
PRIMA-1;5608-24-2
|
|
| PubChem CID |
52918385
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
313.8±17.0 °C at 760 mmHg
|
|
| 闪点 |
143.6±20.9 °C
|
|
| 蒸汽压 |
0.0±1.5 mmHg at 25°C
|
|
| 折射率 |
1.537
|
|
| LogP |
0.61
|
|
| tPSA |
49.77
|
|
| 氢键供体(HBD)数目 |
1
|
|
| 氢键受体(HBA)数目 |
4
|
|
| 可旋转键数目(RBC) |
3
|
|
| 重原子数目 |
14
|
|
| 分子复杂度/Complexity |
236
|
|
| 定义原子立体中心数目 |
0
|
|
| SMILES |
O=C1C2([H])C([H])([H])C([H])([H])N(C([H])([H])C2([H])[H])C1(C([H])([H])O[H])C([H])([H])OC([H])([H])[H]
|
|
| InChi Key |
BGBNULCRKBVAKL-UHFFFAOYSA-N
|
|
| InChi Code |
InChI=1S/C10H17NO3/c1-14-7-10(6-12)9(13)8-2-4-11(10)5-3-8/h8,12H,2-7H2,1H3
|
|
| 化学名 |
2-(hydroxymethyl)-2-(methoxymethyl)-1-azabicyclo[2.2.2]octan-3-one
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (12.55 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (12.55 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (12.55 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 100 mg/mL (501.88 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 5.0188 mL | 25.0941 mL | 50.1882 mL | |
| 5 mM | 1.0038 mL | 5.0188 mL | 10.0376 mL | |
| 10 mM | 0.5019 mL | 2.5094 mL | 5.0188 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Recruitment | interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT04990778 | Withdrawn | Drug: Eprenetapopt Drug: Venetoclax |
Recurrent Mantle Cell Lymphoma Refractory Mantle Cell Lymphoma |
M.D. Anderson Cancer Center | November 30, 2021 | Phase 2 |
Inhibition of TrxR1in vitroby APR-246.Cell Death Dis.2013 Oct 24;4:e881. |
|---|
siRNA knockdown of TrxR1 inhibits APR-246-induced cell death.Cell Death Dis.2013 Oct 24;4:e881. |
siRNA knockdown of TrxR1 inhibits generation of ROS induced by treatment with APR-246.Cell Death Dis.2013 Oct 24;4:e881. |
Inhibition of TrxR1 activity in living cells.Cell Death Dis.2013 Oct 24;4:e881. |
|---|
|
|