| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
Plasmodium
|
|---|---|
| 体外研究 (In Vitro) |
从链霉菌中分离出来的子囊霉素在体外抑制免疫反应,对小鼠混合淋巴细胞的 IC50 为 0.55 nM。 [1] Ascomycin 通过形成 FKBP12-FK520-钙调磷酸酶三元复合物抑制钙调磷酸酶,IC50 为 49 nM。此外,FK520 还可加速神经再生速度并促进神经突生长。 [2] 通过阻断这种双功能蛋白的伴侣活性,子囊霉素表现出抗疟作用。 [3]
Ascomycin/FR-900520和FR-900523是从吸水链霉菌亚种的培养液中分离出来的新型中性大环内酯类免疫抑制剂。烧前7238号。其分子式分别为C43H69NO12和C42H67NO12。这些化合物在体外抑制了免疫反应。FR-900520和FR-900523对小鼠混合淋巴细胞反应的IC50值分别为0.55 nM和1.6 nM。[1] 聚酮FK506(他克莫司)和FK520(Ascomycin)是强效的免疫抑制剂,通过形成FKBP12-FK506/520钙调神经磷酸酶三元复合物来抑制钙调神经酶。它们在细胞培养和神经系统疾病动物模型中也具有钙调神经磷酸酶非依赖性神经再生特性。基于FKBP12-FK506-钙调磷酸酶复合物的晶体结构,我们推断FK506或FK520的13-和15-甲氧基对抑制钙调磷酸酶很重要,但对与FKBP12的结合不重要。通过对FK520基因簇的基因修饰,我们产生了FK520的13-和15-脱甲氧基类似物,它们在其中一个或两个位置含有氢、甲基或乙基而不是甲氧基。这些类似物与FKBP12紧密结合,具有降低钙调神经磷酸酶抑制和免疫抑制特性,并增强细胞培养中的神经突起生长。[2] |
| 体内研究 (In Vivo) |
子囊霉素(3.2 mg/kg,肌肉注射)明显延长大鼠同种异体皮肤移植物的存活时间。 [1] 将 50 或 100 μM 浓度的子囊霉素注入大鼠海马,对印防己毒素引起的癫痫发作具有抗惊厥作用。 [4]
本研究检查了钙调神经磷酸酶(蛋白磷酸酶2B)抑制剂子囊霉素对苦味毒海马内微透析诱导的癫痫发作的潜在体内抗惊厥作用。在建立了个体苦味毒发作阈值后,通过微透析探针以10、50和100微摩的浓度将子囊霉素持续微灌注到大鼠海马中。单独微量灌注子囊霉素期间未观察到行为或脑电图影响。低浓度(10微摩)的ascomycin并不能预防苦味毒素的发作,然而,50和100微摩ascomycin显示出抗癫痫作用,分别完全抑制了41.7%和75%的研究动物的发作。微灌注100微摩的ascomycin显著缩短了平均发作持续时间和平均发作次数(P<0.01)。钙调神经磷酸酶活性可能参与了导致苦味毒诱导的癫痫发作的生化变化。目前的研究结果为磷酸化/去磷酸化机制参与癫痫发作的发展提供了额外的体内证据,表明钙调神经磷酸酶调节可能是寻找新的抗惊厥药物的一种可能策略[4]。 |
| 酶活实验 |
PPI酶测定[3]
重组麦芽糖结合蛋白(MBP)-PfFKBP35-His6在大肠杆菌中产生,并通过连续的镍螯合物和离子交换色谱法纯化至均一性,如其他地方所述。通过使用标准蛋白酶偶联的assa评估其在没有或存在药物的情况下的PPIs活性。简而言之,用分光光度法测量了仅在反式构象下被胰凝乳蛋白酶切割的生色肽底物的顺反式转化。酶浓度为0.25μmol/L,测定缓冲液由50 mmol/L HEPES和100 mmol/L NaCl(pH 8.0)组成,测定温度为0°C(以尽量减少非酶背景异构化)。在包含药物的试验中,它们以1-μL的体积加入,体积为所需浓度的1000倍(终浓度,0.05-5μmol/L),在DMSO中制备。仅用一微升溶剂作为对照。PPI酶活性的IC50值由相应的剂量反应曲线以图形方式确定 伴侣检测[3] MBP-FKBP-His6的制备和纯化如别处所述。猪心线粒体柠檬酸合酶和牛肝硫氰酸盐的热变性基本上如别处所述。简而言之,将柠檬酸合酶(1.5μmol/L单体)在43°C下在40 mmol/L HEPES(pH 7.5)中孵育30分钟,并使用石英微葡萄膜在岛津UV-1601PC分光光度计中监测360 nm处吸光度的增加,从而测量变性过程中的聚集。将Rhodanese(4.4μmol/L)在44°C下在40 mmol/L磷酸钠(pH 8.0)中孵育30分钟,并监测柠檬酸合酶的聚集情况。如结果所述,评估了额外成分对聚集的影响。 |
| 细胞实验 |
生长抑制试验[3]
为了评估子囊霉素/FK520及其类似物对在0.8%寄生虫血症和2%红细胞压积下培养的恶性疟原虫异步寄生的人红细胞的影响,在96孔平底微量滴定板中补充了适当化合物的RPMI 1640培养基中生长72小时。药物从DMSO的储备溶液中稀释到培养基中,然后在微量滴定板的孔中连续稀释2倍,直至亚抑制浓度。孵育后,使用Makler等人基于寄生虫乳酸脱氢酶的测定法确定化合物对寄生虫生长的影响。为每种药物构建了剂量反应曲线。IC50值由相应的剂量反应曲线以图形方式确定。 培养寄生虫对化合物的代谢[3] 通过用5μmol/L的18-烯-20-氧杂-FK520和13dM(Me)-18-烯-20-恶-FK520化合物处理恶性疟原虫培养物48小时,评估寄生虫代谢为其他形式的能力(∼IC30)。将培养物转移到微量离心管中并离心,然后将颗粒在-70°C下冷冻,为高分辨率质谱分析做准备,如其他地方所述。以相同方式处理但暴露于子囊霉素/FK520或13dM(Me)-FK520的细胞作为对照。 |
| 动物实验 |
Recipient WKA rats transplanted with F344 skin allografts.
~32 mg/kg 5 days a week. i.m. Ascomycin was dissolved in Ringer and perfused continuously throughout the experiment in all the animals on different days at 10, 50 and 100 μM concentrations in a random order, following the same protocol for Ringer's solution and picrotoxin administration in the control experiments (Table 1). Each dose was administered once in each animal with resting periods between experiments of at least one week during a total period of 2–3 months. Threshold control experiments were performed on all animals to ensure that no permanent modification had been induced in the duration or number of seizures using the same picrotoxin dose. After finishing ascomycin administration, frequent 3 h EEG controls (2–3 times a week,) with simultaneous video recording were performed in all animals without probe introduction, in order to monitor possible long-term effects of ascomycin and the picrotoxin/ascomycin combination. |
| 毒性/毒理 (Toxicokinetics/TK) |
5282071 mouse LD intraperitoneal >100 mg/kg Journal of Antibiotics, Series A., 15(231), 1962
5282071 mouse LD intraperitoneal >100 mg/kg Journal of Antibiotics., 41(1592), 1988 [PMID:2461926] |
| 参考文献 | |
| 其他信息 |
Ascomycin is a macrolide that is produced by the fermentation of Streptomyces hygroscopicus and exhibits strong immunosuppressant properties. It has a role as an immunosuppressive agent, an antifungal agent and a bacterial metabolite. It is a macrolide, an ether, a lactol and a secondary alcohol.
Ascomycin has been reported in Streptomyces ascomycinicus, Streptomyces hygroscopicus, and Streptomyces clavuligerus with data available. See also: ... View More ... The polyketide macrolactone FK506 inhibits the growth of Plasmodium falciparum in culture and the enzymatic (peptidyl-prolyl cis-trans isomerase [PPIase]) and chaperone activities of a recently identified P. falciparum FK506-binding protein (PfFKBP35). However, the potent immunosuppressive properties of FK506 exclude it from consideration as an antimalarial drug. We describe the antimalarial actions of the related compound FK520 and a number of its nonimmunosuppressive analogues. All compounds were shown to be strong inhibitors of parasite growth, regardless of their immunosuppressive potency. Although some of the compounds inhibited the PPIase activity of recombinant PfFKBP35, they all inhibited the chaperone activity of this bifunctional protein. These findings suggest that the antimalarial effects of this class of drug may be mediated via inhibition of the chaperone activity rather than via the enzymatic activity of PfFKBP35. Elucidating the precise intracellular functions of PfFKBP35 may facilitate the design of more potent inhibitors that retain their specificity for parasite target protein. [3] It is possible, however, that the effects of the compounds are not mediated directly through inhibition of the chaperone activity of PfFKBP35 per se but, rather, through an indirect effect caused by loss of activity of a specific, essential parasite protein whose activity is dependent on PfFKBP35. For example, hFKBP52 has been implicated in the targeted movement of steroid receptors to their sites of action in the nucleus. The C-terminal region of hFKBP52, which contains 3 TPR motifs, directs its association with the steroid receptor heterocomplex, whereas its N-terminal PPIase domain directs its interaction with dynein, the microtubule-associated motor protein involved in retrograde transport. Interestingly, the interaction of the hFKBP52 PPIase domain with dynein is independent of hFKBP52’s intrinsic PPIase activity. This arrangement of an N-terminal PPIase domain followed by a C-terminal tripartite TPR domain is strikingly similar to the domain architecture of PfFKBP35. Work in our laboratory is currently focused on identifying intracellular binding partners for PfFKBP35, because this may well hold the key to elucidating the role of the protein in the parasite and the precise mechanism of action of these drugs. By further dissecting the mode of action of this class of drugs (18-ene-20-oxa-FK520 and 13-dM(Me)-18-ene-20-oxa-FK520 in particular), more potent derivatives could be designed that retain their specificity for the parasite protein. [3] This study reports on the previously uninvestigated in vivo effect of ascomycin on picrotoxin-induced seizures. We have found that ascomycin shows anticonvulsant effect against picrotoxin seizures when perfused into the rat hippocampus at 50 and 100 μM concentrations. No effects were observed with a 10 μM dose. Previous studies have been performed in order to determine the pro- or anticonvulsant effect of several calcineurin inhibitors (Moia et al., 1994, Suzuki et al., 2001, Sanchez et al., 2005), however, they have been limited by the complications of systemic administration such as blood–brain barrier transport and brain tissue distribution. The present study is the first to use direct application to the hippocampus of awake rats, thus allowing the maximum inhibitor concentration at the seizure focus rather than distributed over the large brain areas. Continuous microperfusion permits also to keep a steady extracellular ascomycin concentration over the microdialysis period, minimizing the effect of individual differences in absorption and clearance of the enzyme inhibitor. However, the possibility of chemical interaction among ascomycin and picrotoxin will have to be excluded in further research. [4] |
| 分子式 |
C43H69NO12
|
|
|---|---|---|
| 分子量 |
792.01
|
|
| 精确质量 |
791.481
|
|
| 元素分析 |
C, 65.21; H, 8.78; N, 1.77; O, 24.24
|
|
| CAS号 |
104987-12-4
|
|
| 相关CAS号 |
104987-11-3 (tacrolimus free base); 109581-93-3 (tacrolimus hydrate);11011-38-4 (Ascomycin)
|
|
| PubChem CID |
5282071
|
|
| 外观&性状 |
White to off-white solid powder
|
|
| 密度 |
1.2±0.1 g/cm3
|
|
| 沸点 |
868.3±75.0 °C at 760 mmHg
|
|
| 熔点 |
153-157ºC
|
|
| 闪点 |
478.9±37.1 °C
|
|
| 蒸汽压 |
0.0±0.6 mmHg at 25°C
|
|
| 折射率 |
1.546
|
|
| LogP |
3.81
|
|
| tPSA |
178.36
|
|
| 氢键供体(HBD)数目 |
3
|
|
| 氢键受体(HBA)数目 |
12
|
|
| 可旋转键数目(RBC) |
6
|
|
| 重原子数目 |
56
|
|
| 分子复杂度/Complexity |
1430
|
|
| 定义原子立体中心数目 |
14
|
|
| SMILES |
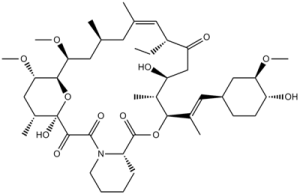
O1[C@]2(C(C(N3C([H])([H])C([H])([H])C([H])([H])C([H])([H])[C@@]3([H])C(=O)O[C@]([H])(/C(/C([H])([H])[H])=C(\[H])/[C@]3([H])C([H])([H])C([H])([H])[C@]([H])([C@@]([H])(C3([H])[H])OC([H])([H])[H])O[H])[C@]([H])(C([H])([H])[H])[C@]([H])(C([H])([H])C([C@]([H])(C([H])([H])C([H])([H])[H])C([H])=C(C([H])([H])[H])C([H])([H])[C@]([H])(C([H])([H])[H])C([H])([H])[C@@]([H])([C@]1([H])[C@]([H])(C([H])([H])[C@@]2([H])C([H])([H])[H])OC([H])([H])[H])OC([H])([H])[H])=O)O[H])=O)=O)O[H] |c:77|
|
|
| InChi Key |
ZDQSOHOQTUFQEM-NURRSENYSA-N
|
|
| InChi Code |
InChI=1S/C43H69NO12/c1-10-30-18-24(2)17-25(3)19-36(53-8)39-37(54-9)21-27(5)43(51,56-39)40(48)41(49)44-16-12-11-13-31(44)42(50)55-38(28(6)33(46)23-34(30)47)26(4)20-29-14-15-32(45)35(22-29)52-7/h18,20,25,27-33,35-39,45-46,51H,10-17,19,21-23H2,1-9H3/b24-18+,26-20+/t25-,27+,28+,29-,30+,31-,32+,33-,35+,36-,37-,38+,39+,43+/m0/s1
|
|
| 化学名 |
(1R,9S,12S,13R,14S,17R,18E,21S,23S,24R,25S,27R)-17-ethyl-1,14-dihydroxy-12-[(E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone
|
|
| 别名 |
|
|
| HS Tariff Code |
2934.99.9001
|
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
|
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (3.16 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (2.63 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (2.63 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.2626 mL | 6.3131 mL | 12.6261 mL | |
| 5 mM | 0.2525 mL | 1.2626 mL | 2.5252 mL | |
| 10 mM | 0.1263 mL | 0.6313 mL | 1.2626 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。