| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10 mM * 1 mL in DMSO |
|
||
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
|
| 靶点 |
ATR ( IC50 = 1 nM ); PI3Kδ ( IC50 = 6.8 μM ); DYRK ( IC50 = 10.8 μM )
|
|
|---|---|---|
| 体外研究 (In Vitro) |
体外活性:AZD6738 是一种口服的基于吗啉代嘧啶的选择性 ATR(共济失调毛细血管扩张和 rad3 相关)激酶抑制剂,IC50 为 1 nM。 ATR是一种丝氨酸/苏氨酸蛋白激酶,在多种癌细胞中表达上调,在DNA修复、细胞周期进程和生存中发挥关键作用;它是由 DNA 复制相关压力期间造成的 DNA 损伤激活的。 AZD6738 具有潜在的抗肿瘤活性。通过口服途径给药后,AZD6738 通过阻断丝氨酸/苏氨酸蛋白激酶 CHK1 的下游磷酸化来选择性抑制 ATR 活性,从而阻止 ATR 介导的信号传导,并导致 DNA 损伤检查点激活的抑制、DNA 损伤修复的破坏,以及诱导肿瘤细胞凋亡。在能够复制的体外模型中,开始 AZD6738 治疗后 70 小时以上,γH2AX 信号持续存在。复制叉停滞可能会破坏 DNA 双链断裂的形成和共济失调毛细血管扩张突变 (ATM) 激酶的激活。作为跨癌细胞系组的单一药物,AZD6738 具有活性。在缺乏 ATM 通路的细胞系中,AZD6738 的敏感性增强。激酶测定:AZD6738 是 ATR 激酶活性的有效抑制剂,对分离的酶的 IC50 为 0.001 μM,对细胞中 ATR 激酶依赖性 CHK1 磷酸化的 IC50 为 0.074 μM。细胞测定:AZD6738 以 30 mM 溶解在 DMSO 中,并在 DMSO 中稀释至所需的工作浓度。对于 AZD6738 剂量反应实验,所有条件和对照的培养基中最终 DMSO 浓度为 0.1%,对于 AZD6738 + 化疗活力实验为 0.05%,对于涉及 0.3 μM 和 1.0 μM 剂量 AZD6738 的所有实验为 0.025%。
|
|
| 体内研究 (In Vivo) |
在 ATM 缺乏但 ATM 不充分的体内模型中,单独使用 AZD6738 治疗可在等效的耐受剂量下显着抑制肿瘤的活性。电离辐射 (IR) 是一种 DNA 损伤诱导剂。当AZD6738和IR一起使用时,观察到消退或抗肿瘤生长抑制活性。在肿瘤组织中,AZD6738 与持续的 γH2AX 染色增加相关。在正常肠道组织或骨髓中,AZD6738 治疗仅短暂增加 γH2AX 染色。
AZD6738和顺铂的组合在NSCLC异种移植物模型中具有疗效,并导致ATM缺陷型NSCLC肿瘤的快速消退 接下来,我们评估了AZD6738单独使用和与顺铂联合使用的体内疗效。AZD6738对小鼠、大鼠和狗的摄食量和体重的影响是剂量限制性的,重复给药后通常会伴有胃肠道萎缩/退行性组织病理学(阿斯利康,个人通讯)。AZD6738导致多个淋巴组织细胞减少,骨髓毒性与外周血中所有细胞系的减少相关。肺泡巨噬细胞略有增加。停止给药后,这些影响得到了恢复。[1] 我们用50mg/kg的AZD6738(PO)治疗携带H460肿瘤的裸鼠,用25mg/kg的AZD6738(PO)处理携带ATM缺陷型H23肿瘤的小鼠,连续14天。小鼠在两周治疗周期的第1天和第8天接受3mg/kg顺铂(IP)。体重减轻是每日单独或与顺铂联合服用50mg/kg AZD6738的剂量限制性毒性。然而,在治疗期间,体重仍在方案指南范围内,研究中的动物在治疗期间的任何时候体重损失均不超过14.3%(图6A)。相反,25mg/kg AZD6738耐受良好,单药组和联合用药组的平均体重(BW)损失分别小于2.7%和4.8%(图6B)。用该组合治疗的小鼠表现出与单独接受顺铂治疗的小鼠相似的BW损失[1]。 50mg/kg AZD6738和顺铂的组合在第14天导致H460异种移植物的平均肿瘤生长抑制率(TGI)为75.5%(与赋形剂相比,P≤0.0001)(图6C)。用联合治疗的肿瘤生长也与单独用顺铂或AZD6738治疗的肿瘤有显著差异(分别为P≤0.01或P≤0.05)。联合治疗的生长延迟为12天(第26天对第14天),尽管在第26天,七个肿瘤中只有一个达到了2000mm3的终点。虽然在单药AZD6738和顺铂治疗组中观察到适度的生长抑制,但生长差异没有统计学意义(P≥0.05)。 [1] 令人惊讶的是,25 mg/kg AZD6738和顺铂的联合使用导致ATM缺陷型H23肿瘤在第29天快速且近乎完全消退(84.8%)(图6D)。肿瘤生长的平均变化与模拟、顺铂和AZD6738治疗组有显著差异(分别为P≤0.001、P≤0.01和P≤0.05)。单独使用顺铂或AZD6738治疗没有显著抑制肿瘤生长(P>0.05)。第29天后,每周观察联合治疗组的小鼠肿瘤再生情况。在接受联合治疗的六只小鼠中,三只分别在第43、64和92天表现出完全的肿瘤消退。在第113天的最终观察中,没有明显的肿瘤迹象。在剩下的三只小鼠中,肿瘤在治疗结束后的3-5周内开始缓慢再生。[1] 我们通过免疫组织化学证实,25mg/kg AZD6738抑制H23异种移植物中的ATR活性。连续8天每天用25mg/kg AZD6738治疗小鼠,第1天和第8天用3mg/kg顺铂、联合用药或赋形剂治疗,并在第8天最后一次给药后6小时收获肿瘤。用AZD6738治疗的小鼠肿瘤显示T1989磷酸化减少(补充图S6),T1989是活性ATR的标志物。 |
|
| 酶活实验 |
AZD6738 是 ATR 激酶活性的有效抑制剂,对分离酶的 IC50 为 0.001 μM,对依赖 ATR 激酶的细胞中 CHK1 磷酸化的 IC50 为 0.074 μM。
ATR和ATM是DNA损伤信号激酶,可磷酸化数千种底物。ATR激酶活性在受损的复制叉和切除的DNA双链断裂(DSBs)处增加。在DSBs处ATM激酶活性增加。ATM已被广泛研究,因为不表达ATM蛋白的共济失调毛细血管扩张症患者是确定的最具放射敏感性的患者。由于ATM不是必需的蛋白质,人们普遍认为ATM激酶抑制剂在临床上会有很好的耐受性。ATR已经被广泛研究,但由于发现ATR是一种必需蛋白,并且人们普遍认为ATR激酶抑制剂在临床上是有毒的,因此进展变得复杂。 |
|
| 细胞实验 |
Ceralasertib (AZD6738) 溶解至 30 mM 浓度后,在 DMSO 中稀释至适当的工作浓度。对于 Ceralasertib (AZD6738) 剂量反应实验,所有条件和对照的培养基中最终 DMSO 浓度为 0.1%;对于 Ceralasertib (AZD6738) + 化疗活力实验,为 0.05%;对于所有涉及 0.3 μM 和 1.0 μM 剂量的 Ceralasertib (AZD6738) 的实验,该比例为 0.025%[1]。
细胞活力测定[1] 细胞在白壁、透明底部96孔板中用指定剂量的AZD6738、顺铂、吉西他滨或其组合处理48小时。使用CellTiter Glo发光细胞存活率测定和Safire2平板读数器评估ATP水平作为存活率的替代指标。在进一步分析之前,对原始数据进行背景发光校正。对于AZD6738治疗,通过对数转换(x=log(x))数据的非线性回归(对数(抑制剂)与可变斜率的反应)在GraphPad Prism 6中生成对数剂量反应曲线,这些数据归一化为未治疗对照的平均值。GI50值定义为Y=50%时的剂量X,从剂量反应曲线推断得出。对于联合治疗,数据被归一化为未经治疗的对照组的平均值。Loewe过量基质是使用Chalice Analyzer Online和平均归一化抑制值生成的。对于AZD6738+顺铂曲线偏移实验,数据被归一化为每种AZD6738治疗条件下0μM顺铂对照的平均值。对数剂量反应曲线是在GraphPad Prism 6中通过对数转换(x=Log(x))的归一化数据的非线性回归(对数(抑制剂)与可变斜率的归一化反应)生成的。IC50值通过Prism 6计算。 免疫印迹[1] 用指定剂量的AZD6738、顺铂、组合或模拟物处理细胞24小时。通过在裂解缓冲液(150 mM NaCl、50 mM Tris-HCL、5 mM NaF、1%吐温20、0.5%IGEPAL CA-630,蛋白酶抑制剂混合物,pH 7.5)中刮取贴壁细胞并在冰上孵育30分钟来产生蛋白质裂解物。对于AZD6738+顺铂实验,将分离的细胞从培养基中沉淀出来并与贴壁细胞裂解物结合。使用4-12%Bis-Tris凝胶进行SDS-PAGE,并使用标准技术进行Western印迹。抗体详细信息见补充方法。在检测到磷酸化蛋白后,在室温下在Restore剥离缓冲液中剥离膜25分钟,并重新检测相应的总蛋白。使用佳能LiDE110扫描仪以24位深度采集印迹图像,并使用ImageJ进行处理(转换为8位,裁剪)。 结晶紫菌落形成和衰老试验[1] 细胞在12孔板中用0.3μM、1μM AZD6738或模拟物处理(一式三份)48小时。处理后,去除AZD6738,细胞在新鲜培养基中再培养2-4天。通过在95%乙醇中用0.5%结晶紫染色来观察菌落形成。图像是用奥林巴斯SZX10立体显微镜和DP26相机拍摄的。未处理的图像被调整大小以包含在图中。实验重复至少三次,以确保结果一致。使用Biovision衰老检测试剂盒评估衰老相关的β-半乳糖苷酶活性。使用Leica DMI3000B倒置显微镜(20X物镜)和DFC420C相机采集图像。未处理的图像被调整大小以包含在图中。 长期细胞存活率的重新接种试验[1] 用0.3μM、1μM AZD6738或模拟物处理细胞48小时。处理后,将细胞以每种条件相同的密度接种在96孔板中(4个重复),并再生长6天。在第8天使用CellTiter Glo发光细胞存活率测定和Safire2平板读数器评估存活率。背景校正数据归一化为未处理对照的平均值. |
|
| 动物实验 |
|
|
| 参考文献 |
|
|
| 其他信息 |
Ceralasertib is under investigation in clinical trial NCT03682289 (Phase II Trial of AZD6738 Alone and in Combination With Olaparib).
Ceralasertib is an orally available morpholino-pyrimidine-based inhibitor of ataxia telangiectasia and rad3 related (ATR) kinase, with potential antineoplastic activity. Upon oral administration, Ceralasertib selectively inhibits ATR activity by blocking the downstream phosphorylation of the serine/threonine protein kinase CHK1. This prevents ATR-mediated signaling, and results in the inhibition of DNA damage checkpoint activation, disruption of DNA damage repair, and the induction of tumor cell apoptosis. In addition, AZD6738 sensitizes tumor cells to chemo- and radiotherapy. ATR, a serine/threonine protein kinase upregulated in a variety of cancer cell types, plays a key role in DNA repair, cell cycle progression and survival; it is activated by DNA damage caused during DNA replication-associated stress. See also: Ceralasertib (annotation moved to). Drug Indication Treatment of lung carcinoma (small cell and non-small cell carcinoma) ATR and ATM are DNA damage signaling kinases that phosphorylate several thousand substrates. ATR kinase activity is increased at damaged replication forks and resected DNA double-strand breaks (DSBs). ATM kinase activity is increased at DSBs. ATM has been widely studied since ataxia telangiectasia individuals who express no ATM protein are the most radiosensitive patients identified. Since ATM is not an essential protein, it is widely believed that ATM kinase inhibitors will be well-tolerated in the clinic. ATR has been widely studied, but advances have been complicated by the finding that ATR is an essential protein and it is widely believed that ATR kinase inhibitors will be toxic in the clinic. We describe AZD6738, an orally active and bioavailable ATR kinase inhibitor. AZD6738 induces cell death and senescence in non-small cell lung cancer (NSCLC) cell lines. AZD6738 potentiates the cytotoxicity of cisplatin and gemcitabine in NSCLC cell lines with intact ATM kinase signaling, and potently synergizes with cisplatin in ATM-deficient NSCLC cells. In contrast to expectations, daily administration of AZD6738 and ATR kinase inhibition for 14 consecutive days is tolerated in mice and enhances the therapeutic efficacy of cisplatin in xenograft models. Remarkably, the combination of cisplatin and AZD6738 resolves ATM-deficient lung cancer xenografts.[1] Ataxia telangiectasia and Rad3-related (ATR) proteins are sensors of DNA damage, which induces homologous recombination (HR)-dependent repair. ATR is a master regulator of DNA damage repair (DDR), signaling to control DNA replication, DNA repair and apoptosis. Therefore, the ATR pathway might be an attractive target for developing new drugs. This study was designed to investigate the antitumor effects of the ATR inhibitor, AZD6738 and its underlying mechanism in human breast cancer cells. Growth inhibitory effects of AZD6738 against human breast cancer cell lines were studied using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (methyl thiazolyl tetrazolium, MTT) assay. Cell cycle analysis, Western blotting, immunofluorescence and comet assays were also performed to elucidate underlying mechanisms of AZD6738 action. Anti-proliferative and DDR inhibitory effects of AZD6738 were demonstrated in human breast cancer cell lines. Among 13 cell lines, the IC50 values of nine cell lines were less than 1 μmol/L using MTT assay. Two cell lines, SK-BR-3 and BT-474, were chosen for further evaluation focused on human epidermal growth factor receptor 2 (HER2)-positive breast cancer cells. Sensitive SK-BR-3 but not the less sensitive BT-474 breast cancer cells showed increased level of apoptosis and S phase arrest and reduced expression levels of phosphorylated check-point kinase 1 (CHK1) and other repair markers. Decreased functional CHK1 expression induced DNA damage accumulation due to HR inactivation. AZD6738 showed synergistic activity with cisplatin. Understanding the antitumor activity and mechanisms of AZD6738 in HER2-positive breast cancer cells creates the possibility for future clinical trials targeting DDR in HER2-positive breast cancer treatment.[2] |
| 分子式 |
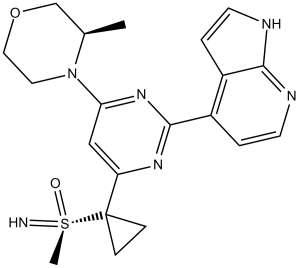
C20H24N6O2S
|
|---|---|
| 分子量 |
412.51
|
| 精确质量 |
412.168
|
| 元素分析 |
C, 58.23; H, 5.86; N, 20.37; O, 7.76; S, 7.77
|
| CAS号 |
1352226-88-0
|
| 相关CAS号 |
1352226-88-0; 1352280-98-8 (formate); 1352226-87-9 (S-isomer); 1352226-97-1 (racemic)
|
| PubChem CID |
54761306
|
| 外观&性状 |
White solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 折射率 |
1.750
|
| LogP |
0.54
|
| tPSA |
116Ų
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
29
|
| 分子复杂度/Complexity |
724
|
| 定义原子立体中心数目 |
2
|
| SMILES |
O=[S@@](C1(CC1)C2=NC(C3=C4C(NC=C4)=NC=C3)=NC(N5CCOC[C@H]5C)=C2)(C)=N
|
| InChi Key |
OHUHVTCQTUDPIJ-JYCIKRDWSA-N
|
| InChi Code |
InChI=1S/C20H24N6O2S/c1-13-12-28-10-9-26(13)17-11-16(20(5-6-20)29(2,21)27)24-19(25-17)15-4-8-23-18-14(15)3-7-22-18/h3-4,7-8,11,13,21H,5-6,9-10,12H2,1-2H3,(H,22,23)/t13-,29-/m1/s1
|
| 化学名 |
imino-methyl-[1-[6-[(3R)-3-methylmorpholin-4-yl]-2-(1H-pyrrolo[2,3-b]pyridin-4-yl)pyrimidin-4-yl]cyclopropyl]-oxo-lambda6-sulfane
|
| 别名 |
AZD6738; AZD-6738; AZD 6738; AZD6738; Ceralasertib; 1352226-88-0; CHEMBL4285417; AZD 6738; BDBM50468001; imino-methyl-[1-[6-[(3R)-3-methylmorpholin-4-yl]-2-(1H-pyrrolo[2,3-b]pyridin-4-yl)pyrimidin-4-yl]cyclopropyl]-oxo-lambda6-sulfane; BDBM60432;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
|
|||
|---|---|---|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 6.67 mg/mL (16.17 mM) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 66.7 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.08 mg/mL (5.04 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 20.8 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.08 mg/mL (5.04 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 配方 4 中的溶解度: 10% DMSO+40% propylene glycol+ddH2O: 10mg/mL 配方 5 中的溶解度: 10 mg/mL (24.24 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 超声助溶. *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.4242 mL | 12.1209 mL | 24.2418 mL | |
| 5 mM | 0.4848 mL | 2.4242 mL | 4.8484 mL | |
| 10 mM | 0.2424 mL | 1.2121 mL | 2.4242 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study to Evaluate the Safety and Pharmacokinetics of Ceralasertib in Combination With Durvalumab in Chinese Patients With Advanced Solid Tumours
CTID: NCT05514132
Phase: Phase 1 Status: Active, not recruiting
Date: 2024-11-08
Inhibition of ATR by AZD6738 inhibits growth of NSCLC cells and induces a DNA damage response.Oncotarget.2015 Dec 29;6(42):44289-305. |
|---|
AZD6738 sensitizes NSCLC cell lines to cisplatin and synergizes strongly with cisplatin in ATM-deficient H23 cells.Oncotarget.2015 Dec 29;6(42):44289-305. |
 The combination of AZD6738 and cisplatin causes accumulation of cells in early S-phase and at the G1/S border.Oncotarget.2015 Dec 29;6(42):44289-305. |
The combination of AZD6738 and cisplatin causes dramatic cell death of ATM-deficient cells independent of the ATM-p53 signaling pathway.Oncotarget.2015 Dec 29;6(42):44289-305. |
|---|
 AZD6738 sensitizes ATM knockdown cells to cisplatin.Oncotarget.2015 Dec 29;6(42):44289-305. |
 AZD6738 potentiates cisplatin efficacy in NSCLC xenografts, and the combination causes rapid regression of ATM-deficient H23 tumors.Oncotarget.2015 Dec 29;6(42):44289-305. |