| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| Other Sizes |
| 靶点 |
BAY2416964 targets indoleamine 2,3-dioxygenase 1 (IDO1) with an IC50 value of 7.8 nM [1]
|
|---|---|
| 体外研究 (In Vitro) |
BAY 2416964(实施例 192)的 IC50 为 4.3 nM,刺激人单核细胞 U937 细胞产生 AHR 调节基因 CYP1A1 [1]。
在重组人IDO1酶活性测定中,BAY2416964 以浓度依赖方式抑制酶活性,IC50值为7.8 nM [1] - 在人A375黑色素瘤细胞中,BAY2416964 抑制IFN-γ诱导的犬尿氨酸生成,IC50值为12 nM,在浓度高达10 μM时无明显细胞毒性 [1] - 在人MDA-MB-231乳腺癌细胞中,BAY2416964 抑制IDO1介导的犬尿氨酸生成,IC50值为9.5 nM,在浓度≤5 μM时不影响细胞活力 [1] - 在人T细胞与树突状细胞共培养实验中,BAY2416964 逆转IDO1诱导的T细胞无反应性,在100 nM浓度下使T细胞增殖增加3.2倍 [1] |
| 体内研究 (In Vivo) |
在携带B16-F10黑色素瘤异种移植瘤的C57BL/6小鼠中,BAY2416964 以10 mg/kg剂量口服给药,每日一次,连续14天,与溶媒对照组相比肿瘤体积减少62% [1]
- 在携带4T1乳腺癌异种移植瘤的BALB/c小鼠中,BAY2416964(20 mg/kg,口服,每日一次,连续12天)抑制肿瘤生长58%,并使血浆犬尿氨酸水平较对照组降低47% [1] - 在B16-F10黑色素瘤荷瘤小鼠中,BAY2416964(10 mg/kg,口服,每日一次)与抗PD-1抗体(10 mg/kg,腹腔注射,每3天一次,连续18天)联合使用时,肿瘤生长抑制率提升至83%,而单独使用抗PD-1抗体的抑制率为41% [1] |
| 酶活实验 |
将重组人IDO1酶与L-色氨酸(底物)在含有抗坏血酸、亚甲基蓝和过氧化氢酶的缓冲液中孵育。加入系列浓度的BAY2416964,混合物在37°C下孵育60分钟。加入三氯乙酸终止反应,随后在95°C下加热15分钟。冷却后加入埃利希试剂,检测492 nm处的吸光度以定量犬尿氨酸生成量,根据浓度-效应曲线计算IC50值 [1]
- 动力学分析中,在固定BAY2416964浓度下,使用不同浓度的L-色氨酸(0.5-100 μM)测定IDO1酶活性。绘制Lineweaver-Burk图以确定抑制模式,结果显示为竞争性抑制,Ki值为3.2 nM [1] |
| 细胞实验 |
将A375和MDA-MB-231细胞接种到96孔板中,过夜培养。用IFN-γ(100 U/mL)预处理细胞24小时以诱导IDO1表达,然后加入系列浓度的BAY2416964。孵育48小时后收集细胞培养上清液,通过高效液相色谱(HPLC)荧光检测法测定犬尿氨酸水平。采用MTT法评估细胞活力,检测570 nm处的吸光度 [1]
- 从人外周血单个核细胞中分离纯化T细胞和树突状细胞,制备T细胞-树突状细胞共培养体系。用脂多糖(LPS)和IFN-γ激活树突状细胞以诱导IDO1表达,然后与CFSE标记的T细胞在BAY2416964存在下共培养。72小时后,通过流式细胞术基于CFSE稀释情况分析T细胞增殖 [1] - 对经IFN-γ处理并暴露于BAY2416964(0.1-10 μM)24小时的A375细胞进行蛋白质印迹分析。细胞裂解物经SDS-PAGE分离后转移至PVDF膜,用抗IDO1、抗STAT1和抗β-肌动蛋白抗体进行探针杂交。采用化学发光检测法显影蛋白条带,结果显示该化合物不影响IDO1蛋白表达,证实其为直接酶抑制剂 [1] |
| 动物实验 |
B16-F10 melanoma cells (5×10^5 cells/mouse) were injected subcutaneously into the right flank of C57BL/6 mice (6-8 weeks old). When tumors reached 100-150 mm³, mice were randomized into groups (n=8 per group). BAY2416964 was formulated in 0.5% methylcellulose and 0.1% Tween 80, and administered orally at 10 mg/kg or 20 mg/kg once daily for 14 days. Tumor volume was measured every 2 days with calipers, and body weight was monitored throughout the study [1]
- For combination therapy, B16-F10 tumor-bearing mice were treated with BAY2416964 (10 mg/kg, p.o., q.d.) plus anti-PD-1 antibody (10 mg/kg, i.p.) every 3 days for 18 days. Control groups received vehicle plus isotype control antibody. At study end, tumors were excised, weighed, and processed for immunohistochemical analysis [1] - 4T1 breast cancer cells (1×10^6 cells/mouse) were injected orthotopically into the mammary fat pad of BALB/c mice. BAY2416964 (20 mg/kg, p.o., q.d.) was administered for 12 days starting 7 days post-inoculation. Blood samples were collected via retro-orbital plexus at study end, and plasma kynurenine and tryptophan levels were measured by HPLC [1] |
| 药代性质 (ADME/PK) |
In mouse pharmacokinetic studies, oral administration of BAY2416964 (10 mg/kg) resulted in a Cmax of 1.8 μg/mL, AUC0-24h of 12.4 μg·h/mL, and oral bioavailability of 78% [1]
- The plasma elimination half-life (t1/2) of BAY2416964 in mice was 4.2 hours after oral dosing and 3.8 hours after intravenous dosing (5 mg/kg) [1] - In vitro human liver microsome stability assay showed BAY2416964 had a half-life of 125 minutes, with low intrinsic clearance (12 μL/min/mg protein) [1] - Plasma protein binding of BAY2416964 was 92% in human plasma and 89% in mouse plasma, as determined by equilibrium dialysis [1] |
| 毒性/毒理 (Toxicokinetics/TK) |
In acute toxicity studies, single oral doses of BAY2416964 up to 200 mg/kg in mice did not cause mortality or significant body weight loss. No gross pathological changes were observed in major organs (liver, kidney, spleen, heart, lungs) [1]
- Repeat-dose toxicity study in mice (14 days, oral administration of 10, 30, or 100 mg/kg/day) showed no dose-related toxicity. Hematological and clinical chemistry parameters were within normal ranges, and histopathological examination revealed no abnormalities in target organs [1] |
| 参考文献 | |
| 其他信息 |
Ilantimod is an orally available formulation containing a small molecule antagonist of the aryl hydrocarbon receptor (AhR; class E basic helix-loop-helix protein 76; bHLHe76) with potential immunomodulating and antineoplastic activities. Upon oral administration, ilantimod specifically binds to AhR, inhibits AhR activation, and prevents AhR-mediated signaling. Abrogation of AhR activation prevents the activation of immune-tolerant dendritic cells (DCs) and regulatory T-cells (Tregs) in the tumor microenvironment (TME). This may restore the immune response against tumor cells. AhR, a member of the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of transcription factors, has important roles in regulating immunity and cellular differentiation. AhR can exhibit both pro-oncogenic and tumor suppressor-like functions depending on the tumor type; therefore, its expression may serve as a negative or positive prognostic factor.
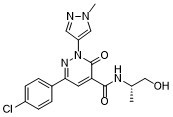
BAY2416964 is a selective, orally active IDO1 inhibitor belonging to the 2-heteroaryl-3-oxo-2,3-dihydropyridazine-4-carboxamide class [1] - The compound exerts anti-tumor effects by inhibiting IDO1-mediated tryptophan catabolism, reducing kynurenine production, and restoring anti-tumor T cell function [1] - BAY2416964 shows potential for the treatment of solid tumors, including melanoma and breast cancer, and enhances the efficacy of immune checkpoint inhibitors such as anti-PD-1 antibodies [1] |
| 分子式 |
C18H18CLN5O3
|
|---|---|
| 分子量 |
387.824
|
| 精确质量 |
387.109
|
| CAS号 |
2242464-44-2
|
| PubChem CID |
135303769
|
| 外观&性状 |
Light yellow to green yellow solid powder
|
| 密度 |
1.42±0.1 g/cm3(Predicted)
|
| LogP |
1.7
|
| tPSA |
99.8
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
5
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
27
|
| 分子复杂度/Complexity |
645
|
| 定义原子立体中心数目 |
1
|
| SMILES |
C[C@@H](CO)NC(=O)C1=CC(=NN(C1=O)C2=CN(N=C2)C)C3=CC=C(C=C3)Cl
|
| InChi Key |
YAGSZKAJPHGVOV-NSHDSACASA-N
|
| InChi Code |
InChI=1S/C18H18ClN5O3/c1-11(10-25)21-17(26)15-7-16(12-3-5-13(19)6-4-12)22-24(18(15)27)14-8-20-23(2)9-14/h3-9,11,25H,10H2,1-2H3,(H,21,26)/t11-/m0/s1
|
| 化学名 |
(S)-6-(4-chlorophenyl)-N-(1-hydroxypropan-2-yl)-2-(1-methyl-1H-pyrazol-4-yl)-3-oxo-2,3-dihydropyridazine-4-carboxamide
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~128.93 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (6.45 mM) in 10% DMSO + 40% PEG300 +5% Tween-80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80+,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.5785 mL | 12.8926 mL | 25.7852 mL | |
| 5 mM | 0.5157 mL | 2.5785 mL | 5.1570 mL | |
| 10 mM | 0.2579 mL | 1.2893 mL | 2.5785 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。