| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 1mg |
|
||
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| Other Sizes |

| 体外研究 (In Vitro) |
苯达莫司汀是一种 DNA 交联剂,可导致抗辅助功能、烷基化和 DNA 片段化。苯达莫司汀特别影响非霍奇金细胞移植和 DNA 修复途径。在 SU-DHL-1 细胞中,苯达莫司汀 (50 μM) 可增强 p53 表达并促进 NOXA 和 p21 (Cip1/Waf1) 基因。苯达莫司汀 (25 μM) 会破坏有丝分裂检查点,导致有丝分裂期间增生 [1]。多发性骨髓瘤 (MM) 细胞系(例如 RPMI-8226 和 8226-LR5)在接触苯达莫司汀时细胞活力较低; 24小时后,这些细胞系的IC25值分别为101.8μM和585.5μM,48小时后分别为51.7和374.3μM。 ..Bendamustine 抑制纺锤体形成检查点并导致 caspase 触发的 MM 细胞死亡 [2]。
|
|---|---|
| 体内研究 (In Vivo) |
DoHH-2、Granta 519 和 RAMOS 模型显示苯达莫司汀(25 mg/kg,IV)对肿瘤细胞增殖的抑制率为 91%、99% 和 95%。此外,利妥昔单抗在 DoHH-2 和 RAMOS 模型中改善了苯达莫司汀的抗癌作用,但在 Granta 519 模型中没有改善 [3]。
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Following a single IV dose of bendamustine hydrochloride Cmax typically occurred at the end of infusion. The dose proportionality of bendamustine has not been studied. Mean recovery of total radioactivity in cancer patients following IV infusion of [14C] bendamustine hydrochloride was approximately 76% of the dose. Approximately 50% of the dose was recovered in the urine and approximately 25% of the dose was recovered in the feces. Urinary excretion was confirmed as a relatively minor pathway of elimination of bendamustine, with approximately 3.3% of the dose recovered in the urine as parent. Less than 1% of the dose was recovered in the urine as M3 and M4, and less than 5% of the dose was recovered in the urine as HP2. The mean steady-state volume of distribution (Vss) of bendamustine was approximately 20-25 L. Steady-state volume of distribution for total radioactivity was approximately 50 L, indicating that neither bendamustine nor total radioactivity are extensively distributed into the tissues. 700 mL/min ... Preclinical radiolabeled bendamustine studies showed that approximately 90% of drug administered was recovered in excreta primarily in the feces. Bendamustine clearance in humans is approximately 700 mL/minute. After a single dose of 120 mg/sq m bendamustine IV over 1-hour the intermediate half life of the parent compound is approximately 40 minutes. The mean apparent terminal elimination half life of M3 and M4 are approximately 3 hours and 30 minutes respectively. Little or no accumulation in plasma is expected for bendamustine administered on Days 1 and 2 of a 28-day cycle. In vitro, the binding of bendamustine to human serum plasma proteins ranged from 94-96% and was concentration independent from 1-50 ug/mL. Data suggest that bendamustine is not likely to displace or to be displaced by highly protein-bound drugs. The blood to plasma concentration ratios in human blood ranged from 0.84 to 0.86 over a concentration range of 10 to 100 ug/mL indicating that bendamustine distributes freely in human red blood cells. In humans, the mean steady state volume of distribution (Vss) was approximately 25 L. Metabolism / Metabolites In vitro data indicate that bendamustine is primarily metabolized via hydrolysis to monohydroxy (HP1) and dihydroxy-bendamustine (HP2) metabolites with low cytotoxic activity. Two active minor metabolites, M3 and M4, are primarily formed via CYP1A2. However, concentrations of these metabolites in plasma are 1/10th and 1/100th that of the parent compound, respectively, suggesting that the cytotoxic activity is primarily due to bendamustine. Results of a human mass balance study confirm that bendamustine is extensively metabolized via hydrolytic, oxidative, and conjugative pathways. ... The detection of mercapturic acid pathway metabolites of bendamustine, namely, cysteine S-conjugates in human bile, which are supposed to subsequently undergo further metabolism /was recently reported/.. In this study, ... the identification and quantitation of consecutive bendamustine metabolites occurring in human bile using authentic reference standards and the synthesis and structural confirmation of these compounds /is described/. Mass spectrometry data along with high-performance liquid chromatography retention data (fluorescence detection) of the synthetic reference standards were consistent with those of the metabolites found in human bile after administration of bendamustine hydrochloride to cancer patients. Analysis of the purified synthetic reference compounds showed a purity of at least 95%. Structural confirmation was achieved by one- and two-dimensional proton as well as carbon-13 NMR spectroscopy and mass spectrometry. A total of 16 bendamustine-related compounds were detected in the bile of patients, 11 of them were recovered as conjugates. Eight conjugates have been structurally confirmed as novel mercapturic acids and sulfoxides. Biliary excretion of the sulfoxides was twice that of the mercapturate precursors. Glutathione S-conjugates of bendamustine have not been detected in bile samples, indicating rapid enzymatic cleavage in humans. Both the lack of glutathione (GSH) conjugates and occurrence of diastereomeric sulfoxides emphasize species-related differences in the GSH conjugation of bendamustine between humans and rats. The total amount recovered in the bile as the sum of all conjugates over the period of 24 hr after dosing averaged 5.2% of the administered dose. In vitro data indicate that bendamustine is primarily metabolized via hydrolysis to metabolites with low cytotoxic activity. In vitro, studies indicate that two active minor metabolites, M3 and M4, are primarily formed via CYP1A2. However, concentrations of these metabolites in plasma are 1/10 and 1/100 that of the parent compound, respectively, suggesting that the cytotoxic activity is primarily due to bendamustine. In vitro studies using human liver microsomes indicate that bendamustine does not inhibit CYP1A2, 2C9/10, 2D6, 2E1, or 3A4/5. Bendamustine did not induce metabolism of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1, or CYP3A4/5 enzymes in primary cultures of human hepatocytes. Biological Half-Life 40 minutes After a single dose of 120 mg/sq m bendamustine IV over 1-hour the intermediate half life of the parent compound is approximately 40 minutes. |
| 毒性/毒理 (Toxicokinetics/TK) |
Hepatotoxicity
Mild and transient elevations in serum aminotransferase levels are found in up to 20% of patients treated with bendamustine, but elevations above 5 times the upper limit of normal occur in less than 3% of patients. The abnormalities are generally transient, unaccompanied by symptoms and rarely require dose modification. Clinically apparent liver injury from bendamustine has been limited to a small number of cases of mild hepatitis with features of hypersensitivity including eosinophilia, rash or other systemic symptoms. Autoantibody formation is uncommon. The course is generally self-limited, but may require corticosteroid therapy for control of symptoms and timely recovery. Bendamustine therapy has also been implicated in causing reactivation of hepatitis B in patients with anti-HBc in serum with or without HBsAg. In several instances patients were also receiving corticosteroids or rituximab, yet had received these without reactivation in the past. Reactivation arose after 2 to 6 cycles of bendamustine chemotherapy, presenting with symptoms accompanied by HBsAg and rising levels of HBV DNA in serum. Reactivation was generally self-limited and patients later became HBsAg negative. In one instance, however, the course was severe and resulted in death from acute liver failure. Likelihood score: C (probable cause of clinically apparent liver injury, some of which is due to reactivation of hepatitis B). Effects During Pregnancy and Lactation ◉ Summary of Use during Lactation No information is available on the use of bendamustine during breastfeeding. Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, especially alkylating agents such as bendamustine. Based on the half-life of the drug and its metabolites, the drug should be eliminated from the milk by 24 to 48 hours after the last dose. The manufacturer recommends that breastfeeding be discontinued during bendamustine therapy and for at least 1 week after the last dose. ◉ Effects in Breastfed Infants Relevant published information was not found as of the revision date. ◉ Effects on Lactation and Breastmilk Some evidence indicates that the closely related drug carmustine can increase serum prolactin. Protein Binding In vitro, the binding of bendamustine to human serum plasma proteins ranged from 94-96% and data suggest that bendamustine is not likely to displace or to be displaced by highly protein-bound drugs. Non-Human Toxicity Values LD50 Rat oral 200-300 mg/kg /Bendamustine hydrochloride/[The Merck Index, Fourteenth Edition (2006) LD50 Rat iv 40 mg/kg /Bendamustine hydrochloride/[The Merck Index, Fourteenth Edition (2006) LD50 Mouse oral 400-500 mg/kg /Bendamustine hydrochloride/[The Merck Index, Fourteenth Edition (2006) LD50 Mouse iv 80 mg/kg /Bendamustine hydrochloride/[The Merck Index, Fourteenth Edition (2006) |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Antineoplastic /Bendamustine hydrochloride/[The Merck Index, Fourteenth Edition (2006) Bendamustine hydrochloride is used for the treatment of chronic lymphocytic leukemia (CLL) and is designated an orphan drug by the US Food and Drug Administration (FDA) for use in this condition. /Included in US product label/ Bendamustine administered as monotherapy is active in rituximab-refractory indolent non-Hodgkin's lymphoma, predominantly in patients with nontransformed or with sensitive disease characteristics. Therefore, bendamustine may be considered a reasonable choice for rituximab-refractory, indolent non-Hodgkin's lymphoma; bendamustine may also may be considered an alternative in patients who are not candidates for radioimmunotherapy due to either patient selection (i.e., a clinical contraindication) or accessibility issues. /Included in US product label/ Treanda for Injection is an alkylating drug indicated for treatment of patients with: Chronic lymphocytic leukemia (CLL). Efficacy relative to first line therapies other than chlorambucil has not been established. Indolent B-cell non-Hodgkin's lymphoma (NHL) that has progressed during or within six months of treatment with rituximab or a rituximab-containing regimen. /Included in US product label/ For more Therapeutic Uses (Complete) data for Bendamustine (8 total), please visit the HSDB record page. Drug Warnings Infections, including pneumonia and sepsis, have been reported in patients receiving bendamustine and have been associated with hospitalization, septic shock, and death. Patients with myelosuppression have increased susceptibility to infection and should be advised to contact their clinician if signs or symptoms of infection occur. In the phase 3 study in patients with chronic lymphocytic leukemia, grade 3 or 4 neutropenia occurred in 24% and febrile neutropenia occurred in 3% of patients receiving bendamustine; red blood cell or platelet transfusions were administered to 20 or less than 1%, respectively, of patients receiving the drug. Leukocytes, platelets, hemoglobin, and neutrophils should be monitored closely in patients with bendamustine-related myelosuppression. In the phase 3 study of bendamustine in chronic lymphocytic leukemia, hemoglobin concentrations and leukocyte and differential counts were monitored weekly and platelet counts were monitored each cycle. Data from this study indicate that blood counts may be expected to reach a nadir during the third week of the treatment cycle; dose delays may be required if recovery to recommended values has not occurred by day 28. Prior to initiation of the next cycle of therapy, the absolute neutrophil count (ANC) should be at least 1000/cu mm and the platelet count at least 75,000/ cu mm. Tumor lysis syndrome has been reported in patients receiving bendamustine in clinical trials and during postmarketing surveillance. The onset generally occurs during the first cycle of bendamustine therapy; without appropriate intervention, acute renal failure and death may occur. Appropriate measures (e.g., adequate hydration; close monitoring of blood chemistries, particularly potassium and uric acid concentrations; use of allopurinol during the first 1-2 weeks of bendamustine therapy) should be used in patients at high risk for tumor lysis syndrome. Infusion reactions, including fever, chills, pruritus, and rash, occur commonly in patients receiving bendamustine. Severe anaphylactic and anaphylactoid reactions have occurred rarely, mainly in the second and subsequent cycles of therapy. Patients should be monitored clinically, and bendamustine should be discontinued if a severe reaction occurs. Patients should be asked about symptoms suggestive of infusion reactions after their first cycle of therapy. A premedication regimen (e.g., antihistamine, antipyretic, and corticosteroid) should be considered during subsequent treatment cycles in patients who experience grade 1 or 2 infusion reactions. Discontinuance of bendamustine therapy should be considered in patients who experience grade 3 or 4 infusion reactions. Patients who experienced grade 3 or worse allergic-type reactions typically were not rechallenged with the drug in the phase 3 study of bendamustine in chronic lymphocytic leukemia. For more Drug Warnings (Complete) data for Bendamustine (15 total), please visit the HSDB record page. Pharmacodynamics No mean changes in QTc interval greater than 20 milliseconds were detected up to one hour post-infusion. |
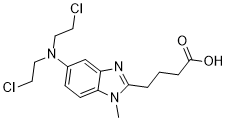
| 分子式 |
C16H21N3O2CL2
|
|---|---|
| 分子量 |
358.26284
|
| 精确质量 |
357.101
|
| CAS号 |
16506-27-7
|
| 相关CAS号 |
Bendamustine hydrochloride;3543-75-7;Bendamustine-d4;Bendamustine-d8;1134803-33-0
|
| PubChem CID |
65628
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
585.2±50.0 °C at 760 mmHg
|
| 闪点 |
307.7±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.599
|
| LogP |
2.9
|
| tPSA |
58.4
|
| 氢键供体(HBD)数目 |
1
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
23
|
| 分子复杂度/Complexity |
380
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN1C2=C(C=C(C=C2)N(CCCl)CCCl)N=C1CCCC(=O)O
|
| InChi Key |
YTKUWDBFDASYHO-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C16H21Cl2N3O2/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23/h5-6,11H,2-4,7-10H2,1H3,(H,22,23)
|
| 化学名 |
4-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]butanoic acid
|
| 别名 |
Bendamustine free base; SDX 105; SDX-105; SDX105; Bendamustina; DD6304600; Bendamustinum; Ribomustin. Brand name: Treanda;
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~279.13 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (6.98 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.7913 mL | 13.9563 mL | 27.9127 mL | |
| 5 mM | 0.5583 mL | 2.7913 mL | 5.5825 mL | |
| 10 mM | 0.2791 mL | 1.3956 mL | 2.7913 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
A Study of Zilovertamab Vedotin (MK-2140) in Combination With Standard of Care in Participants With Relapsed or Refractory Diffuse Large B-Cell Lymphoma (rrDLBCL) (MK-2140-003)
CTID: NCT05139017
Phase: Phase 2/Phase 3 Status: Recruiting
Date: 2024-11-21
|
|
|