| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 5mg |
|
||
| 10mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| 1g |
|
||
| Other Sizes |
|
| 靶点 |
p110α (IC50 = 4 nM); p110α-H1047R (IC50 = 4.6 nM); p110α-E545K (IC50 = 5.7 nM); p110γ (IC50 = 5 nM); p110δ (IC50 = 7 nM); p110β (IC50 = 75 nM); mTOR (IC50 = 20.7 nM); mTORC1; mTORC2; Autophagy
|
|---|---|
| 体外研究 (In Vitro) |
Dactolisib (BEZ235) 以 ATP 竞争方式有效抑制 PI3K。 250 nM 剂量的 dactolisib (BEZ235) 显着降低了 p70S6K(一种 mTOR 激活激酶)的磷酸化水平。由于 mTOR 的激酶结构域与 IA 类 PI3K 的激酶结构域高度相似,Dactolisib (BEZ235) 也会导致 S235/S236P-RPS6 水平降低,IC50 为 6.5 nM。生化 mTOR K-LISA 测定(IC50,20.7 nM)用于证明 Dactolisib (BEZ235) 的抗 mTOR 活性[1]。 Dactolisib (BEZ235) 对 HCT116、DLD-1 和 SW480 细胞系的 IC50 分别为 14.36.4、9.01.5 和 12.01.6 nM[2]。
NVP-BEZ235以ATP竞争方式有效抑制PI3K。NVP-BEZ235特异性阻断细胞中的PI3K通路。NVP-BEZ235对Akt下游效应器和mTOR的影响。 NVP-BEZ235具有很强的抗增殖活性。[1] 体外NVP-BEZ235处理人CRC细胞系会降低细胞增殖,但对凋亡没有影响。体外NVP-BEZ235治疗人CRC细胞系可导致持续的mTORC1和mTORC2抑制,但短暂的PI3K阻断。 体外NVP-BEZ235治疗人CRC细胞系的疗效不取决于PIK3CA突变状态[2]。 |
| 体内研究 (In Vivo) |
在散发性 PIK3CA 野生型 CRC 的 GEM 模型中,Dactolisib (BEZ235)(45 mg/kg,口服)治疗可诱导结肠肿瘤消退[2]。 Dactolisib (BEZ235) 以 45 mg/kg 的剂量口服给 MENX 大鼠(每组 n=2),1 或 6 小时后处死动物。与用 PEG 治疗的大鼠相比,P-AKT 和 P-S6 的免疫染色显示,在 Dactolisib (BEZ235) 给药 6 小时后,这两种蛋白(尤其是 P-S6)显着减少。 Dactolisib (BEZ235) 治疗的大鼠的垂体腺瘤的蛋白质组谱与治疗后 6 小时安慰剂治疗的大鼠的肿瘤显着不同[3]。
达考替尼(BEZ235,NVP-BEZ 235)是一种口服PI3K抑制剂,具有良好的耐受性抗肿瘤活性[1] Dactolisib (BEZ235, NVP-BEZ 235)的药代动力学特性最初是在携带PC3M肿瘤的裸鼠体内进行评估的。在50 mg/kg的剂量下,Dactolisib (BEZ235, NVP-BEZ 235)在血浆中迅速出现,0.5小时的Cmax为1.68μmol/L,C24h为0.03μmol/L。在肿瘤组织中,1小时时达到的Cmax(Tmax)为2.05 nmol/g,24小时后降至0.23 nmol/g(图5A)Dactolisib(BEZ235,NVP-BEZ 235)从肝脏中相对较快地消除。对肿瘤组织中S473P-Akt水平的体内分析显示,给药后1小时达到最大抑制作用(对应于肿瘤Cmax),治疗后16小时仍观察到持续抑制作用,给药24小时后,四个肿瘤中的两个几乎完全恢复到基础水平(图5B)。基于这项研究的药代动力学模拟表明,当以50mg/kg的剂量每天给药或以25mg/kg的剂量每天两次给药时,3至5天内将达到稳态水平。剂量方案之间的差异在于预测的肿瘤峰值水平(分别为2.6和1.6 nmol/g),而贯穿水平几乎保持相当(分别为0.53和0.60μmol/L)。考虑到这种药代动力学模拟,以25mg/kg口服NVP-BEZ235的剂量对携带PC3M肿瘤的动物进行慢性治疗,每天两次。使用该时间表,观察到肿瘤生长受到统计学上的显著抑制,治疗10天后的最终T/C值为22%(图5C)。NVP-BEZ235对体重增加的非统计学显著影响(图5C)以及研究过程中没有动物死亡的事实表明,该治疗具有良好的耐受性。通过肿瘤提取物的蛋白质印迹或肿瘤切片的免疫染色检测到,抗肿瘤作用与最后一剂后1或18小时肿瘤组织中S473P-Akt的抑制密切相关(图5D)。这些时间点的化合物浓度在1小时时为1.32nmol/g,在18小时时为0.51nmol/g,接近稳态峰值水平的预测值(见上文)。 Dactolisib (BEZ235, NVP-BEZ 235)体内治疗散发性CRC的GEM模型可导致持续的mTORC1和mTORC2抑制,但短暂的PI3K阻断[2] 为了检查体内达可替尼(BEZ235、NVP-BEZ 235)治疗对PI3K和mTOR信号传导的影响,在治疗第5天和第28天最后一次给药后一小时采集的结肠肿瘤中进行了蛋白质印迹分析。分别对p-AKTThr308、p-S6Ser240/244和p-AKTSer473的水平进行了蛋白质斑点分析,作为PI3K、mTORC1和mTORC2通路激活的替代品。与对照组相比,NVP-BEZ235治疗的小鼠肿瘤中p-AKTSer 473和p-S6Ser240/244的水平持续降低。稀释剂(图4A和4B)。这些发现得到了肿瘤免疫组织化学的证实(图4C)。与我们的体外研究一样,在治疗后五天观察到p-AKTThr308水平的初始下降,但在28天后恢复正常(图4A和4B)。综上所述,这些结果表明,体内NVP-BEZ235治疗可持续抑制mTORC1和mTORC2,但会短暂阻断PI3K。 |
| 酶活实验 |
PI3Kα、β 和 δ蛋白由 p85 的 iSH2 结构域与完整蛋白 p110 融合组成,但同样缺少最后 20 个氨基酸。生成删除了前 144 个氨基酸的全长 PI3K 蛋白。为了便于纯化,每个构建体在克隆到 pBlue-Bac4.5 或 pVL1393 质粒(具体取决于所研究的亚型)之前,先与 COOH 末端 His 标签融合。然后,使用供应商建议的技术来生产适当的重组杆状病毒和蛋白质,将各种载体与 BaculoGold WT 基因组 DNA 共转染。 Kinase-Glo 测定用于评估 BEZ235 抑制 PI3K 的能力。 384 孔黑色板用于激酶反应。然后将 PI3K 蛋白(分别为 10、25、10 和 150 nM 的 p110、p110、p110 和 p110)添加到每个孔中,然后在每个孔中加载 50 L 测试项目(在 90% DMSO 中)和 5 L 含有 10 g/mL PI 底物(L-磷脂酰肌醇;Avanti Polar Lipids)的反应缓冲液。添加在反应缓冲液中制备的 5 L 1 M ATP 以开始反应,然后孵育 60(对于 p110、p110 和 p110)或 120(对于 p110)分钟。添加 10 L Kinase-Glo 缓冲液结束反应。然后使用 Synergy 2 读数器检查板的发光情况。 384 孔黑色板用于激酶反应。然后将 PI3K 蛋白(分别为 10、25、10 和 150 nM 的 p110α、p110β、p110δ 和 p110γ)添加到每个孔中,然后在每个孔中加载 50 μL 测试项目(在 90% DMSO 中)和 5 μL 反应缓冲液含有 10 μg/mL PI 底物(L-磷脂酰肌醇;Avanti Polar Lipids)。添加在反应缓冲液中制备的 5 μL 1 μM ATP 以开始反应,然后孵育 60 分钟(对于 p110α、p110β 和 p110δ)或 120 分钟(对于 p110γ)。添加 10 μL Kinase-Glo 缓冲液结束反应。然后使用 Synergy 2 读数器检查板的发光情况。
|
| 细胞实验 |
人 CRC 细胞系 HCT116(PIK3CA 突变体;激酶结构域位于 H1047R)、DLD-1(PIK3CA 突变体;螺旋结构域位于 E545K)和 SW480(PIK3CA 野生型)和同基因 DLD-1 PIK3CA 突变体以及野生型类型细胞维持在含有 10% FBS 和 1 × 青霉素/链霉素的 DMEM 中。为了考虑不同的生长动力学,细胞以不同的初始密度铺板(HCT116:3 103 细胞/孔,DLD-1:5.5 103 细胞/孔,SW480:4.5 103 细胞/孔,DLD-1 PIK3CA 突变体:7 103 细胞/孔,和DLD-1 PIK3CA野生型:9 103个细胞/孔)。 16小时后,将BEZ235以逐渐增加的浓度添加到细胞中,并且每24小时更换一次含有该药物的生长培养基。根据制造商的建议,使用比色 MTS 检测 CellTiter 96® AQueous One Solution 细胞增殖检测在药物治疗开始后 48 小时和初次接种后 16 小时测量细胞活力。将药物处理后的细胞活力与生长 48 小时的未处理细胞进行比较。在进行蛋白质印迹分析之前,将细胞在不使用 BEZ235 或使用最大抑制剂量 (500 nM) 的情况下进行 2、6、24 或 48 小时。
|
| 动物实验 |
Mice: An oral gavage of 45 mg/kg of BEZ235 in 10% 1-methyl-2-pyrrolidone/90% PEG 300 is administered daily for 28 days to tumor-bearing Apc CKO mice or a control vehicle alone (n = 8) for treatment. Based on evidence from the literature, the recommended dose of BEZ235 is 40–50 mg/kg body weight, which has been shown to be safe and effective in treating murine tumor models. Tumor-bearing mice are sacrificed one hour after the last dose of the drug, in accordance with pharmacokinetic studies showing that the tissue concentration of NVP-BEZ235 reaches its peak one hour after administration. Utilizing calipers to measure the width, length, and height of the colonic tumor, tumors are harvested for immunohistochemistry and western blot analysis.
Rats: MENX-affected rats used. In MENX rats, BEZ235 is tested at doses of 20, 30, and 45 mg/kg. The dose of 20 mg/kg is used for further studies because the two higher doses result in a weight loss of greater than 10% after 10 days of treatment. For MRI studies, BEZ235 (20 mg/kg) or a placebo (PEG) are given orally once daily to MENX-affected rats aged 7 to 8 months who have sizeable adenomas but are otherwise healthy. Establishment of Xenograft Tumors, Efficacy Studies, Compound Preparation, and Analytics [1] Establishment of tumors, group randomization, tumor, and body weight recording during efficacy studies were described elsewhere. Antitumor activity is expressed as %T/C (mean increase of tumor volumes of treated animals divided by the mean increase of tumor volumes of control animals multiplied by 100) and/or as tumor regression (%Reg) calculated as [(mean tumor volume at the start of treatment - mean tumor volume) / (mean tumor volume at the start of treatment)] × 100. Data are presented as mean ± 1 SE. Comparisons between groups and vehicle control group were done using either one-way ANOVA or ANOVA on ranks followed by Dunnett's tests when data were respectively either normally distributed or not. For all tests, the level of significance was set at P < 0.05. Calculations were done using SigmaStat version 2.03. Dactolisib (BEZ235, NVP-BEZ 235) (free base) was formulated in NMP/polyethylene glycol 300 (10/90, v/v). Solutions (5 mg/mL) were prepared fresh each day of dosing as follows: the powder was dissolved in NMP on sonication, and the remaining volume of polyethylene glycol 300 was added. The application volume was 10 mL/kg. For analytics, frozen tissues were minced and then homogenized in an equal volume of ice-cold PBS using a Polytron homogenizer (IKA). After acetonitrile precipitation and centrifugation, supernatants were analyzed by reverse-phase high-performance liquid chromatography/UV on a Merck-Hitachi/LaChrom equipment including a Nucleosil 100-5 C18 column. Samples were then eluted with a linear gradient of 10% to 90% (v/v) acetonitrile in water containing 0.05% (v/v) trifluoroacetic acid over a period of 20 min at a flow rate of 1 mL/min. The compounds were detected by UV absorbance at 340 nm, and concentrations were determined by the external standard method using peak heights. In vivo treatment of a GEM model for sporadic CRC [2] Apc CKO mice were treated with Adeno-Cre and followed by optical colonoscopy, as previously described [11]. As a colonoscopic metric for tumor size, the Tumor Size Index (TSI) was calculated as (tumor area/colonic lumen area)×100 (%). Tumor-bearing mice were randomly assigned to treatment with either control vehicle alone (n = 8) or 45 mg/kg body weight Dactolisib (BEZ235, NVP-BEZ 235) in 10% 1-methyl-2-pyrrolidone/90% PEG 300 (n = 8) by daily oral gavage for 28 days. The treatment dose was chosen based on literature indicating that 40–50 mg/kg body weight Dactolisib (BEZ235, NVP-BEZ 235) effectively treats murine tumor models without adverse effects. Based on pharmacokinetic studies demonstrating maximal tissue concentration one hour after NVP-BEZ235 administration, tumor-bearing mice were sacrificed one hour after final treatment dose. Colonic tumor volume was assessed using calipers (width×length×height) and tumors were harvested for both western blot analysis and immunohistochemistry. |
| 药代性质 (ADME/PK) |
The pharmacokinetic properties of NVP-BEZ235 were originally evaluated in PC3M tumor-bearing nude mice. At a dose of 50 mg/kg, NVP-BEZ235 appeared rapidly in plasma with a Cmax of 1.68 μmol/L at 0.5 h and a C24h of 0.03 μmol/L. In the tumor tissue, the Cmax attained was 2.05 nmol/g at 1 h (Tmax), which decreased to 0.23 nmol/g after 24 h (Fig. 5A). NVP-BEZ235 is eliminated relatively quickly from the liver. In vivo analysis of the S473P-Akt levels in the tumor tissue revealed that maximum inhibition was obtained 1 h after dosing (corresponding to the tumor Cmax), and persistent inhibition was observed still 16 h after treatment, with almost complete recovery to basal levels obtained in two of four tumors at 24 h after dose (Fig. 5B). Pharmacokinetic simulation based on this study revealed that steady-state levels would be achieved between 3 and 5 days, when dosing either at 50 mg/kg given daily or at 25 mg/kg given twice daily. Differences between the dosage regimens would reside in the predicted tumor peak levels (2.6 versus 1.6 nmol/g, respectively) whereas through levels would remain almost comparable (0.53 versus 0.60 μmol/L, respectively). Taking into consideration this pharmacokinetic simulation, chronic treatment of PC3M tumor-bearing animals was done at 25 mg/kg p.o. NVP-BEZ235 twice daily. Using this schedule, a statistically significant inhibition of tumor growth was observed, with a final T/C value of 22% after 10 days of treatment (Fig. 5C). The treatment was well tolerated as concluded from the nonstatistically significant effect of NVP-BEZ235 on body weight gain (Fig. 5C) and by the fact that none of the animals died during the course of the study. The antitumor effect was well correlated with the inhibition of S473P-Akt in the tumor tissue 1 or 18 h after the last dose detected either by Western blotting of tumor extracts or by immunostaining of tumor sections (Fig. 5D). Compound concentrations at these time points were 1.32 nmol/g at 1 h and 0.51 nmol/g at 18 h, close to the predicted values for steady-state peak levels (see above).[1]
|
| 参考文献 |
|
| 其他信息 |
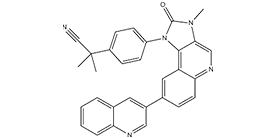
Dactolisib is an imidazoquinoline that is 3-methyl-2-oxo-2,3-dihydro-1H-imidazo[4,5-c]quinoline substituted at position 1 by a 4-(1-cyanoisopropyl)phenyl group and at position 8 by a quinolin-3-yl group. A dual PI3K/mTOR inhibitor used in cancer treatment. It has a role as an EC 2.7.1.137 (phosphatidylinositol 3-kinase) inhibitor, a mTOR inhibitor and an antineoplastic agent. It is an imidazoquinoline, a nitrile, a member of quinolines, a ring assembly and a member of ureas.
Dactolisib has been used in trials studying the treatment of Cancer, Solid Tumor, Renal Cancer, Breast Cancer, and Cowden Syndrome, among others. Dactolisib is an orally bioavailable imidazoquinoline targeting the phosphatidylinositol 3 kinase (PI3K) and the mammalian target of rapamycin (mTOR), with potential antineoplastic activity. Dactolisib inhibits PI3K kinase and mTOR kinase in the PI3K/AKT/mTOR kinase signaling pathway, which may result in tumor cell apoptosis and growth inhibition in PI3K/mTOR-overexpressing tumor cells. Activation of the PI3K/mTOR pathway promotes cell growth, survival, and resistance to chemotherapy and radiotherapy; mTOR, a serine/threonine kinase downstream of PI3K, may also be activated independent of PI3K. The phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin inhibitor (mTOR) pathway is often constitutively activated in human tumor cells, providing unique opportunities for anticancer therapeutic intervention. NVP-BEZ235 is an imidazo[4,5-c]quinoline derivative that inhibits PI3K and mTOR kinase activity by binding to the ATP-binding cleft of these enzymes. In cellular settings using human tumor cell lines, this molecule is able to effectively and specifically block the dysfunctional activation of the PI3K pathway, inducing G(1) arrest. The cellular activity of NVP-BEZ235 translates well in in vivo models of human cancer. Thus, the compound was well tolerated, displayed disease stasis when administered orally, and enhanced the efficacy of other anticancer agents when used in in vivo combination studies. Ex vivo pharmacokinetic/pharmacodynamic analyses of tumor tissues showed a time-dependent correlation between compound concentration and PI3K/Akt pathway inhibition. Collectively, the preclinical data show that NVP-BEZ235 is a potent dual PI3K/mTOR modulator with favorable pharmaceutical properties. NVP-BEZ235 is currently in phase I clinical trials.[1] Purpose: To examine the in vitro and in vivo efficacy of the dual PI3K/mTOR inhibitor NVP-BEZ235 in treatment of PIK3CA wild-type colorectal cancer (CRC). Experimental design: PIK3CA mutant and wild-type human CRC cell lines were treated in vitro with NVP-BEZ235, and the resulting effects on proliferation, apoptosis, and signaling were assessed. Colonic tumors from a genetically engineered mouse (GEM) model for sporadic wild-type PIK3CA CRC were treated in vivo with NVP-BEZ235. The resulting effects on macroscopic tumor growth/regression, proliferation, apoptosis, angiogenesis, and signaling were examined. Results: In vitro treatment of CRC cell lines with NVP-BEZ235 resulted in transient PI3K blockade, sustained decreases in mTORC1/mTORC2 signaling, and a corresponding decrease in cell viability (median IC(50) = 9.0-14.3 nM). Similar effects were seen in paired isogenic CRC cell lines that differed only in the presence or absence of an activating PIK3CA mutant allele. In vivo treatment of colonic tumor-bearing mice with NVP-BEZ235 resulted in transient PI3K inhibition and sustained blockade of mTORC1/mTORC2 signaling. Longitudinal tumor surveillance by optical colonoscopy demonstrated a 97% increase in tumor size in control mice (p = 0.01) vs. a 43% decrease (p = 0.008) in treated mice. Ex vivo analysis of the NVP-BEZ235-treated tumors demonstrated a 56% decrease in proliferation (p = 0.003), no effects on apoptosis, and a 75% reduction in angiogenesis (p = 0.013). Conclusions: These studies provide the preclinical rationale for studies examining the efficacy of the dual PI3K/mTOR inhibitor NVP-BEZ235 in treatment of PIK3CA wild-type CRC.[2] |
| 分子式 |
C30H23N5O
|
|---|---|
| 分子量 |
469.5365
|
| 精确质量 |
469.19026
|
| 元素分析 |
C, 76.74; H, 4.94; N, 14.92; O, 3.41
|
| CAS号 |
915019-65-7
|
| 相关CAS号 |
Dactolisib Tosylate;1028385-32-1; 915019-65-7; 2319647-83-9 (HCl)
|
| PubChem CID |
11977753
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
701.0±70.0 °C at 760 mmHg
|
| 熔点 |
288-289°C
|
| 闪点 |
377.8±35.7 °C
|
| 蒸汽压 |
0.0±2.2 mmHg at 25°C
|
| 折射率 |
1.705
|
| LogP |
3.72
|
| tPSA |
76.5
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
36
|
| 分子复杂度/Complexity |
872
|
| 定义原子立体中心数目 |
0
|
| SMILES |
N#CC(C)(C)C1C=CC(N2C3C(=CN=C4C=3C=C(C3C=C5C(C=CC=C5)=NC=3)C=C4)N(C)C2=O)=CC=1
|
| InChi Key |
JOGKUKXHTYWRGZ-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3
|
| 化学名 |
2-Methyl-2-[4-[3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydroimidazo[4,5-c]quinolin-1-yl]phenyl]propionitrile.
|
| 别名 |
BEZ 235; BEZ235; BEZ-235; Dactolisib; NVPBEZ235; NVP-BEZ235; BEZ235; NVP-BEZ 235; 2-methyl-2-(4-(3-methyl-2-oxo-8-(quinolin-3-yl)-2,3-dihydro-1H-imidazo[4,5-c]quinolin-1-yl)phenyl)propanenitrile; NVP-BEZ 235; NVP-BEZ-235; NVP-BEZ235
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO: ~0.01 mg/mL (0.02 mM)
Water: <1 mg/mL (slightly soluble or insoluble) DMF: 18 mg/mL warming (38.33 mM) |
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 0.52 mg/mL (1.11 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 5.2 mg/mL澄清的DMSO储备液加入到400 μL PEG300中,混匀;再向上述溶液中加入50 μL Tween-80,混匀;然后加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 0.52 mg/mL (1.11 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 5.2 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 View More
配方 3 中的溶解度: NMP+polyethylene glycol 300 (10/90, v/v): 30mg/mL 配方 4 中的溶解度: 12.5 mg/mL (26.62 mM) in 50% PEG300 50% Saline (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 5 中的溶解度: ≥ 1.1 mg/mL (2.34 mM) (饱和度未知) in 10% 1-Methyl-2-pyrrolidinone 90% PEG300 (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1297 mL | 10.6487 mL | 21.2974 mL | |
| 5 mM | 0.4259 mL | 2.1297 mL | 4.2595 mL | |
| 10 mM | 0.2130 mL | 1.0649 mL | 2.1297 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
| NCT Number | Status | Interventions | Conditions | Sponsor/Collaborators | Start Date | Phases |
| NCT03373903 | Active Recruiting |
Drug: BEZ235 Drug: BEZ235 plus everolimus (RAD001) |
Respiratory Tract Infections | Restorbio Inc. | November 15, 2017 | Phase 2 |
| NCT04584710 | Active Recruiting |
Drug: RTB101 Drug: Placebo |
Covid19 | Restorbio Inc. | October 13, 2020 | Phase 2 |
Effect of the dual PI3K/mTOR inhibitor NVP-BEZ235 on 3D organotypic cultures of rat primary NFPA cells. |
Expression of DEFB1 in human NFPAs and immortalized gonadotroph cells. Clin Cancer Res. 2015 Jul 15;21(14):3204-15. |
Effect of the dual PI3K/mTOR inhibitor NVP-BEZ235 in vivo. |
Expression ofDEFB1in human NFPAs and immortalized gonadotroph cells.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
Role ofDEFB1in NET cell lines.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
Expression ofDefb1,Tnfrsf10b, andBcl2a1in rat pituitary adenoma tissues after NVP-BEZ235 treatmentin vivo.Clin Cancer Res.2015 Jul 15;21(14):3204-15. |
|
 |
 |