| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 靶点 |
Natural product; PXR
|
|---|---|
| 体外研究 (In Vitro) |
Byakangelicin (Byn)/Umb混合物在大脑中的积累[1]
大脑中Umb的积累也被评估为其他化合物/Umb重量比的增加。静脉注射Umb和Umb混合物(Byakangelicin (Byn)/Umb、Dec/Umb、Del/Umb、Nod/Umb和Ang/Umb)后,Umb剂量为4 通过荧光成像观察Umb在每个分离器官中的定位(其他化合物/Umb的重量比为20)。如图3A所示,用Byn/Umb混合物治疗后,脑和肺中的Umb FI显著升高。然而,在Byn/Umb给药后,胰腺的FI没有明显增加。与其他化合物混合物相比,Nod/Umb在胰腺中的Umb积累更高。还对每个器官中的FI进行了定量分析。图3B显示,每个器官(脑、肺和胰腺)中Umb的归一化FI分别为8.9±4.7、8.1±9.1,分别为8.2±6.2(×10−3)。Byn/Umb(其他化合物/Umb的重量比为20)在脑和肺中的FI分别为37.4±0.1和58.0±29.5(×10−3)。用Byn/Umb治疗的小鼠脑和肺中的FI分别是单独用Umb治疗小鼠的4.2倍和7.2倍。为了确认通过离体荧光监测确定的器官中的Umb水平是否与常规分析方法相关,在从每个器官中提取Umb后,使用荧光光谱仪评估了Umb的物理量。图3C显示了使用乙酸乙酯从每个器官提取的组织裂解物中的相对FI。正如预期的那样,与单独注射Umb的小鼠相比,注射Byn/Umb的老鼠的大脑和肺部显示出更大量的Umb(图3C)。然而,从用Ang/Umb治疗的小鼠的脑和肺中提取的Umb量与从单独注射Umb的小鼠的大脑和肺中提取物的量相似,这与离体成像结果不一致。这可归因于Umb在复杂提取过程中的不稳定性。Byn和Nod与Umb的混合物分别显示大脑和胰腺中的Umb水平显著升高。这一结果与图3B中的离体监测密切相关。与仅使用Umb相比,Byn/Umb重量比为20的Byl/Umb混合物导致大脑中Umb积聚增加2.4±0.1倍。 通过离体荧光成像研究Cur和Dox的生物分布[1] 为了研究Byakangelicin(Byn)是否可以调节不同类型活性化合物的生物分布,将Cur和Dox与Byakangelcin(Byn>)的混合物静脉注射给小鼠。Cur是一种众所周知的多酚,存在于姜黄中,对神经炎症性疾病和阿尔茨海默病具有治疗作用(Mishra和Palanivelu,2008)。如图S2A所示,随着Cur浓度的增加,Cur的荧光信号相应增强。Cur与Byn的混合物(Byn/Cur)显示出与单独使用Cur相似的FI,这表明用Cur和Byn/Cur-混合物处理的组织中Cur的量可以分别通过溶液中Cur含量来测量(图S2B)。Dox是一种代表性的抗癌药物,用于治疗包括神经母细胞瘤在内的多种类型的肿瘤(MacDiarmid等人,2016)。然而,Dox穿过血脑屏障的渗透性差限制了其对脑肿瘤的抗癌作用(Rousselle等人,2000,Seol等人,2014)。因此,开发有前景的多克斯脑给药系统受到了极大的关注。Cur和Dox都可以被外部光荧光激发,在体内给药后可以很容易地进行监测(Kang等人,2016b,Motlagh等人,2016)。图4A显示了施用Cur混合物(Byn/Cur、Dec/Cur和Ang/Cur)后Cur在脑和肺中的荧光信号。与仅使用Cur和其他化合物混合物相比,Byn/Cur混合物在脑和肺中显示出明显强的FI。如图4B所示,还对每个器官中的FI进行了定量分析。Cur、Byn/Cur、Dec/Cur和Ang/Cur(其他化合物/Cur的重量比=50)在大脑中的归一化FI分别为3.6±1.2、8.3±2.1、5.8±1.7,此外,肺中Cur、Byn/Cur、Dec/Cur和Ang/Cur(其他化合物/Cur的重量比=50)的归一化FI分别为6.2±2.7、46.5±2.0、6.7±2.7,18.7±17.1,分别。有趣的是,与仅使用Cur相比,使用Byn/Cur混合物在肺部观察到Cur的积聚超过7倍。在我们之前的研究中,通过离体荧光成像确定的Cur生物分布与从组织中提取的Cur的质谱分析结果高度相关(Kang等人,2016a)。因此,组织中Cur的强荧光强度可能表明Cur在组织中的实际积累。然而,有必要检查不同时间间隔的Cur分布,以阐明Cur在不同组织中的最大积累(Wei等人,2017,Zhang等人,2019)。图4C显示了Dox在三个不同器官中的积累。正如预期的那样,Dox与Byn的混合物在大脑中的Dox积累量在其他化合物混合物中最高。脑中Dox、Byn/Dox、Dec/Dox和Nod/Dox(其他化合物/Dox的重量比=10)的归一化FI分别为2.6±0.7、15.0±6.8、9.0±6.5,2.9±0.6,分别如图4D所示。总的来说,与没有Byn的注射相比,Byn与Umb、Cur和Dox的混合物分别将它们在大脑中的积累提高了4.2倍、2.3倍和5.7倍。为了研究Byn是否能促进Eb的渗透,将Eb与Byn混合,并用混合物治疗小鼠。图S3显示,服用Byn/Eb混合物后,脑、肺和胰腺中Eb的FI与单独服用Eb后相似。这一结果清楚地表明,Byn在不破坏BBB的情况下增强了选定活性化合物的脑积累。 LPS诱导的炎症模型中Cur in的脑积累[1] 为了检查Cur在LPS诱导的小鼠炎症模型中的定位,将Cur和Byakangelicin (Byn)静脉注射给小鼠5天。在第5天给药Cur和Byakangelicin (Byn)后,还向小鼠腹腔注射LPS。24小时后,分离并固定大脑以制备冠状切片。使用荧光显微镜在大脑(图5A)和共聚焦显微镜在海马(图5B)的冠状切片中观察到Cur的积聚。与仅使用Cur相比,Byn/Cur治疗后大脑中的Cur积累显著增强。不仅在大脑,而且在海马区都观察到强烈的绿色荧光信号。综上所述,这些结果表明,在LPS诱导的炎症模型中,Byn允许Cur在大脑和海马中大量积累。 Byakangelicin (Byn)在LPS诱导的炎症模型中的抗炎作用 为了研究Byakangelicin (Byn)在LPS诱导的炎症模型中的抗炎作用,在给予Cur和Byn/Cur后,通过ELISA测定脑匀浆和血清中的细胞因子水平。对照组、缓冲组、Cur组和Byn/Cur组的TNF-α含量分别为14.5±1.4、25.6±1.6、14.5±1.3,分别为11.2±2.7 pg/mg(图6A)。与Cur组相比,Byn/Cur组脑匀浆中TNF-α的水平略有降低。此外,在Cur和Byn/Cur组中观察到IL-1β水平的显著差异。对照组、缓冲组、Cur组和Byn/Cur组的IL-1β含量分别为1.8±1.1、7.6±1.2、3.6±1.2 0.8, 分别为2.3±0.7 pg/mg(图6B)。为了比较Cur和Byn/Cur的全身抗炎作用,分析了血清中TNF-α的水平,如图6C所示。LPS给药24小时后,血清中的TNF-α水平为30.1±4.5 pg/ml。然而,Cur和Byn/Cur组的血清中TNF-α水平分别降至23.4±2.3和4.5±4.5 pg/ml。值得注意的是,Byn/Cur组的TNF-α水平与未注射LPS的小鼠(对照组)几乎相似。这一结果清楚地表明,Byn/Cur的增强积累可以对小鼠神经炎症模型产生成功的抗炎作用。 |
| 体内研究 (In Vivo) |
通过离体荧光成像研究Umb的生物分布[1]
图1A显示了来自A.gigas的六种化合物的化学结构。在这些化合物中,基于香豆素的化合物Umb被选为示踪化合物,因为它在325 nm波长处具有很强的吸光度,在495 nm波长处有荧光(Simkovitch等人,2016,Vasconcelos等人,2009)。还检查了其他生物活性化合物Cur和Dox,以监测体内生物分布。作为对照染料,Eb也被给予小鼠以检查其大脑定位。将这些单一化合物和这些化合物的混合物注射到正常小鼠和神经炎症小鼠模型中后,测定其在分离器官中的生物分布。特别是,活性化合物(Umb、Cur和Dox)在每个器官中的生物分布可以使用离体荧光成像仪器直接可视化,而不需要耗时复杂的提取过程,如图1C所示。通过腹腔注射LPS建立神经炎症小鼠模型(Walker等人,2013,Wang等人,2014)。为了评估Cur和Byakangelicin (Byn)混合物的抗炎作用,将这些药物静脉注射到小鼠体内五次(图1B)。给药后,分别通过荧光显微镜和细胞因子ELISA检测Cur在脑中的积聚及其抗炎反应。 图2A显示了水溶液中单一化合物和其他化合物/Umb混合物的荧光图像。只有在Umb存在的情况下才观察到明显的荧光信号(图2A)。Umb、Byakangelicin (Byn)、Dec/Umb和Ang/Umb的归一化FI分别为100.9±5.8、101.3±3.3、97.3±8.8,102.9±6.0,表明其他化合物对Umb荧光的影响可以忽略不计。在小鼠静脉注射160或320 mg/kg剂量的Umb后,通过荧光成像监测Umb的器官分布。在脑、肺和胰腺中观察到强烈的荧光信号(图2B)。众所周知,Umb对糖尿病和神经退行性疾病具有治疗作用,这与Umb的生物分布有关(Naowaboot等人,2015,Ramu等人,2016,Subramaniam和Ellis,2013,Wang等人,2015)。为了研究Umb的生物分布是否可以通过与来自A.gigas的其他化合物共同给药来改变,将Umb与Byakangelicin (Byn)、Dec和Ang的混合物静脉注射到小鼠体内。然后通过荧光成像观察Umb在每个器官中的定位。2分钟后,与使用其他混合物相比,使用Byn/Umb混合物后,在大脑和肺部观察到更强的Umb荧光信号(图2C)。在用Byn/Umb和Ang/Umb混合物治疗的小鼠中观察到胰腺中的荧光信号显著降低。然而,如图S1A所示,静脉注射后,Byakangelicin (Byn)、Dec和Ang单独在任何器官中都显示出可忽略的荧光信号。提取后,还对每个器官中的荧光信号进行了定量分析。在用另一种化合物给药Umb后,脑、肺和胰腺中的Umb水平明显降低(图2D)。单独使用Umb、Byn/Umb、Dec/Umb和Ang/Umb的归一化FI分别为382.1±69.0、630.5±104.4、363.8± 104.6, 大脑中为503.2±64.0(×10−3),177.4±23.8,132.3±4.5,163.4±36.5,肺部为344.3±272.9(×10−3)。在与Byn一起服用Umb后,脑和肺中Umb的积累量最大。如图S1B所示,从每个器官中提取后,Byn、Dec和Ang的FI可以忽略不计。为了检查观察到的Umb积累增加是否是由化合物混合物给药后BBB的破坏介导的,在化合物治疗后也给药了Eb。图S1C清楚地显示了脑中Eb的积累较差。该结果清楚地表明,在施用化合物混合物后,BBB保持完整。 |
| 酶活实验 |
体外荧光成像[1]
将DMSO中的单一化合物(Umb、Byakangelicin (Byn)、Dec和Ang)储备用注射缓冲液(含13.5%(v/v)kolliphor的PBS)稀释至终浓度为50 用注射缓冲液以50µg/ml的恒定Umb浓度稀释DMSO中的Umb混合物(Byakangelicin(Byn)/Umb、Dec/Umb和Ang/Umb)(其他化合物/Umb的重量比为1)。将样品装入96孔黑板后,分别在360和490 nm的激发和发射波长下使用Lago-X分析FI。 |
| 细胞实验 |
脑裂解物和血清中细胞因子水平的测定[1]
为了测量大脑中促炎细胞因子的水平,将大脑在PBS溶液中均质化。在4°C下以13000 rpm离心10分钟后,收集上清液并储存在-80°C下直至使用。为了制备血清,将分离的血液在2000×g下离心15分钟。根据制造商的方案,使用BCA蛋白检测试剂盒对每个样品中的蛋白质含量进行定量。使用小鼠ELISA试剂盒测定各脑匀浆和血清中TNF-α和IL-1β的含量。使用酶标仪在450nm处测量吸光度。 |
| 动物实验 |
Ex vivo fluorescence imaging of Umb [1]
For the injection of compound mixtures, ICR mice were injected intravenously with Umb and compound mixtures (weight ratio of other compound/Umb = 1) in injection buffer at an Umb dose of 80 mg/kg, and left for 2 min. After 2 min, the mice were euthanized, and organs were isolated and washed with PBS solution. Fluorescent images of each organ were analyzed using Lago-X at an excitation and emission wavelength of 360 nm and 490 nm, respectively. The total flux within a whole organ was quantitatively analyzed after subtracting background signals using the following formula: total flux in tissue from treated mice - total flux in tissue from untreated mice. A. gigas extract and single compounds (Byakangelicin (Byn), Ang, and Dec) were also injected into the mice tail vein at doses of 120 mg/kg (extract) and 40 mg/kg (single compound). After incubation for 15 min, the FI in each organ was monitored using Lago-X at an excitation/emission wavelength of 360/490 nm, respectively. To determine whether the blood-brain barrier (BBB) was disrupted by Umb or the compound mixtures, ICR mice were injected intravenously with freshly prepared Umb and Umb mixtures (other compound/Umb weight ratio of 1) at an Umb dose of 40 mg/kg. After incubation for 15 min, a 1% Eb solution in PBS was injected intravenously. After an additional 15 min of incubation, the mice were euthanized and fluorescent images of Eb in each tissue were acquired using an in vivo imaging system (IVIS) instrument at an excitation and emission wavelength of 535 and 705 nm, respectively. Umb mixtures (Byakangelicin (Byn)/Umb, Dec/Umb, Del/Umb, Nod/Umb, and Ang/Umb) dissolved in DMSO were diluted with injection buffer at a final Umb concentration of 0.5 mg/ml, and then injected into ICR mice at an Umb dose of 4 mg/kg at different other compound/Umb weight ratios of 0, 1, and 20. After 2 min, the organs were collected and analyzed using Lago-X at an excitation and emission wavelength of 360 nm and 490 nm, respectively. The total flux in each organ was analyzed using the Lago-X software. To quantify the amount of Umb in different organs (brain, lung, and pancreas), Umb was extracted using ethyl acetate after the isolated organs were homogenized according to previous study (Kang et al., 2016a). Briefly, the organs were homogenized in PBS solution and the homogenate was then mixed with a solution of 10% SDS and ethyl acetate to give a volume ratio of homogenate:10% SDS:ethyl acetate of 1:0.5:5. After vortexing the mixture for 10 min, the sample was centrifuged at 12,000 rpm for 3 min. The supernatant was then collected and dried at room temperature. After the dried tissue extracts were re-dissolved in DMSO, the FIs in samples were measured at an excitation and emission wavelength of 325 and 495 nm using a fluorospectrophotometer (Molecular Devices, CA, USA). The FI of Umb in DMSO (0–500 ng/ml) served as standard for quantification. Ex vivo fluorescence imaging of Cur and Dox in brain [1] To measure the FI of Cur and Byakangelicin (Byn)/Cur solutions, each sample was prepared in injection buffer at a Cur concentration of 1 µg/ml (at a weight ratio of Byn/Cur of 2). After samples were loaded into 96-well black plates, the FIs were analyzed using an in vivo imaging system at an excitation and emission wavelength of 430 and 509 nm, respectively. Cur mixtures with other compounds, including Byakangelicin (Byn)/Cur, Dec/Cur, and Ang/Cur, were prepared at various weight ratios (other compound/Cur weight ratios of 0, 20, and 50). Dox mixtures with other compounds, including Byn/Dox, Dec/Dox, and Nod/Dox, were also prepared at other compound/Dox weight ratios of 0, 5, and 10, respectively. For injection, the Cur and Dox mixtures in DMSO were mixed with kolliphor and PBS at 1.5:1.5:7.0 (volume ratio of DMSO: kolliphor: PBS), according to a previous study (Zhang et al., 2015). Freshly prepared Cur (1.6 mg/kg) and Dox (5 mg/kg) mixtures were injected intravenously into ICR mice, respectively. After incubation for 2 min, the brain and lung were isolated and washed with PBS solution. The fluorescent signal in each organ was analyzed using IVIS instrument at an excitation/emission wavelength of 430/509 nm for Cur, and at an excitation/emission wavelength of 465/583 nm for Dox, respectively. As a control, Eb was mixed with Byn at Byn/Eb weight ratios of 0, 0.5, 1, 2, 20, and 50. The Byn/Eb mixtures in injection buffer were intravenously administered at an Eb dose of 1.6 mg/kg. After 2 min, the mice were perfused with saline, the organs were collected and analyzed using IVIS instrument at excitation/emission wavelength of 535/ 705 nm. LPS-induced neuro-inflammation model [1] A neuro-inflammation animal model was established, as previously described (Walker et al., 2013, Wang et al., 2014). C57/BL6 mice were randomly divided into four groups: a normal group treated with injection buffer (Control); a control group pre-treated with injection buffer before LPS injection (Buffer); a Cur group pre-treated with Cur before LPS injection (Cur); and a Byakangelicin (Byn)/Cur group pre-treated with Byn/Cur before LPS injection (Byn/Cur). All solutions were prepared fresh on the day of administration. The schedule for Cur injection was slightly modified based on a previous study (Kawamoto et al., 2013, Wang et al., 2014). For pre-treatment, a solution of Cur and Byn/Cur in injection buffer was injected into the tail vein once per a day at a Cur dose of 12 mg/kg and Byakangelicin (Byn) doses of 0 and 24 mg/kg for 5 days, respectively. Thirty minutes after injection on the 5th day, the mice in groups of Buffer, Cur, and Byn/Cur were injected intraperitoneally with LPS (0.83 mg/kg). After 24 h of LPS injection, the mice were anesthetized with isoflurane and sera were collected from mice. After perfusion, the brains were collected and cut into half. The brains in left-hand side were used to prepare a homogenate, whereas brains in right-hand side were used for brain imaging. For confocal imaging, the right-hand brain sections were fixed in 3.7% formaldehyde in PBS solution for 48 h, and then dehydrated in 15 and 30% (w/v) sucrose in PBS solution. The samples were frozen in optimum cutting temperature (OCT) and a coronal section with a width of 10 µm was made using a cryostat. After the samples were stained with DAPI, the FIs of Cur and DAPI in each tissue slice were observed using fluorescence microscopy and confocal microscopy, respectively. |
| 参考文献 |
|
| 其他信息 |
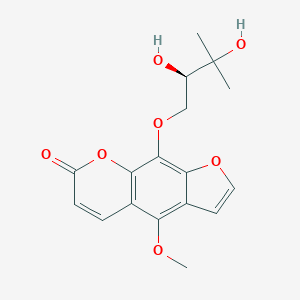
Byakangelicin is a member of psoralens.
Byakangelicin has been reported in Angelica japonica, Heracleum grandiflorum, and other organisms with data available. Background: The elucidation of the biological roles of individual active compounds in terms of their in vivo bio-distribution and bioactivity could provide crucial information to understand how natural compounds work together as treatments for diseases. Purpose: We examined the functional roles of Byakangelicin (Byn) to improve the brain accumulation of active compounds, e.g., umbelliferone (Umb), curcumin (Cur), and doxorubicin (Dox), and consequently to enhance their biological activities. Methods: Active compounds were administered intravenously to mice, with or without Byn, after which organs were isolated and visualized for their ex vivo fluorescence imaging to determine the bio-distribution of each active compound in vivo. For the in vivo bioactivity, Cur, either with or without Byn, was administered to a lipopolysaccharide (LPS)-induced neuro-inflammation model for 5 days, and its anti-inflammatory effects were examined by ELISA using a brain homogenate and serum. Results: We successfully demonstrated that the levels of active compounds (Umb, Cur, and Dox) in the brain, lung, and pancreas were greatly elevated by the addition of Byn via direct ex vivo fluorescence monitoring. In addition, sufficient accumulation of the active compound, Cur, greatly reduced LPS-induced neuro-inflammation in vivo. Conclusion: Byn could serve as a modulator to allow improved brain accumulation of diverse active compounds (Umb, Cur, and Dox) and enhanced therapeutic effects.[1] |
| 分子式 |
C17H18O7
|
|---|---|
| 分子量 |
334.324
|
| 精确质量 |
334.105
|
| 元素分析 |
C, 61.07; H, 5.43; O, 33.50
|
| CAS号 |
482-25-7
|
| 相关CAS号 |
(Rac)-Byakangelicin;19573-01-4
|
| PubChem CID |
10211
|
| 外观&性状 |
White to yellow solid powder
|
| 密度 |
1.4±0.1 g/cm3
|
| 沸点 |
571.5±50.0 °C at 760 mmHg
|
| 熔点 |
123-124℃
|
| 闪点 |
299.4±30.1 °C
|
| 蒸汽压 |
0.0±1.7 mmHg at 25°C
|
| 折射率 |
1.613
|
| LogP |
1.62
|
| tPSA |
102.27
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
503
|
| 定义原子立体中心数目 |
1
|
| SMILES |
CC(C)([C@@H](COC1=C2C(=C(C3=C1OC(=O)C=C3)OC)C=CO2)O)O
|
| InChi Key |
PKRPFNXROFUNDE-LLVKDONJSA-N
|
| InChi Code |
InChI=1S/C17H18O7/c1-17(2,20)11(18)8-23-16-14-10(6-7-22-14)13(21-3)9-4-5-12(19)24-15(9)16/h4-7,11,18,20H,8H2,1-3H3/t11-/m1/s1
|
| 化学名 |
9-[(2R)-2,3-dihydroxy-3-methylbutoxy]-4-methoxyfuro[3,2-g]chromen-7-one
|
| 别名 |
Biacangelicin; Byakangelicin; Byankagelicine; Byak-angelicin; Bjacangelicin; Bjakangelicin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~50 mg/mL (~149.56 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (7.48 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: 2.5 mg/mL (7.48 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浊液; 超声助溶。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.9911 mL | 14.9557 mL | 29.9115 mL | |
| 5 mM | 0.5982 mL | 2.9911 mL | 5.9823 mL | |
| 10 mM | 0.2991 mL | 1.4956 mL | 2.9911 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
|
|
|