| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
A study of 4 male and 4 female Sprague-Dawley rats treated intramuscularly with (14)C-ceftiofur (2 mg/kg bw) revealed that 55% of the administered dose was excreted in the urine and about 30% in the GI tract and feces. The major urinary metabolite was desfuroylceftiofur (DFC). The metabolism of ceftiofur was similar in calves administered (14)C-ceftiofur (2 mg/kg bw) via the i.m. route. Unmetabolized ceftiofur was also present in the urine (4.4-21% of total radioactivity). A group of Sprague-Dawley rats (7/sex) received single oral doses of (14)C-ceftiofur (200 mg/kg bw) in a comparative study with calves. Approximately 55% of the total dose was recovered in the urine and the rest was present in the feces and GI tract. Plasma concentration at 6 hr was 1 mg/kg and trace amounts of ceftiofur were present in all tissues (i.e. liver, muscle and fat). The highest residue levels (0.7 mg/kg) were present in kidney. A study of lactating cows treated with (14)C-ceftiofur (2.3 mg/kg bw/day for 5 days) revealed that 32-38% of the radioactivity was present in the milk as free metabolites. The major metabolite was desfuroylceftiofur cysteine disulfide representing 7-9% of the total radioactivity. No parent compound was detected in the milk. A study of im administration of (14)C-ceftiofur in a bull revealed that 55% of the administered dose was excreted in the urine and approximately 30% in the GI tract and feces. The initial metabolite in both urine and plasma was desfuroylceftiofur. HPLC analysis of radioactive metabolites was similar to the results found in the rat studies. A number of metabolites were produced, the major metabolite (87% of total urinary metabolites) being desfuroylceftiofur acetamide conjugates. No parent compound was observed in the urine. For more Absorption, Distribution and Excretion (Complete) data for CEFTIOFUR (13 total), please visit the HSDB record page. Metabolism / Metabolites A study of 4 male and 4 female Sprague-Dawley rats treated intramuscularly with (14)C-ceftiofur (2 mg/kg bw) revealed that 55% of the administered dose was excreted in the urine and about 30% in the GI tract and faeces. The major urinary metabolite was desfuroylceftiofur (DFC). The metabolism of ceftiofur was similar in calves administered (14)C-ceftiofur (2 mg/kg bw) via the i.m. route. Unmetabolized ceftiofur was also present in the urine (4.4-21% of total radioactivity). A group of Sprague-Dawley rats (7/sex) received single oral doses of (14)C-ceftiofur (200 mg/kg bw) in a comparative study with calves. Approximately 55% of the total dose was recovered in the urine and the rest was present in the feces and GI tract. ... The major urinary metabolite was ceftiofursulfoxide cysteine thioester. HPLC analysis of metabolites of (14)C-ceftiofur formed by arochlor-induced rat liver S-9 fractions in vitro revealed that desfuroylceftiofur was the major metabolite. Low doses (119 mg/kg bw) of ceftiofur were completely metabolized within 15 minutes. Higher doses (857 mg/kg bw) were converted to desfuroylceftiofur after 60 minutes of incubation. A study in 8-week old Sprague-Dawley rats (7/sex) treated with (14)C-ceftiofur (800 mg/kg bw/day) by oral gavage for 5 days revealed several urinary metabolites, including desfuroylceftiofur, ceftiofur sulfoxide, and cysteine disulfide. For more Metabolism/Metabolites (Complete) data for CEFTIOFUR (15 total), please visit the HSDB record page. Biological Half-Life Six Friesian calves (3/sex) were treated with ceftiofur according to different protocols including one single im and iv injection at 1 mg/kg bw, and 5 i.m. injections at 1 mg/kg bw at 24 hr intervals. ... The half life (0.07 hr) was short due to rapid metabolism to desfuroylceftiofur. The t1/2 of desfuroylceftiofur after im and iv administration were similar (9.7 and 8.6 hr, respectively). A study of 4 calves (sex and breed unspecified) administered ceftiofur intramuscularly daily for 4 days at 2 dose levels (2.2 or 4.4 mg/kg bw/day) demonstrated a plasma half life of 3.5 hr. ... Plasma half life of the metabolite desfuroylceftiofur was 9.7 h after im administration. A study of 4- to 5-month old Yorkshire-Hampshire pigs (6/sex) treated with 3 daily im injections of (14)C-ceftiofur (5.2 mg/kg bw) produced similar results to those observed in rats and cattle. ... The half life of desfuroylceftiofur was 13.5 hr after im treatment and 12.2 hr after iv treatment. /Desfuroylceftiofur/ |
|---|---|
| 其他信息 |
Ceftiofur is a third generation cephalosporin antibiotic, first described in 1987, and now used in veterinary medicine. It is marketed by pharmaceutical company Zoetis as Excenel, and is the active ingredient in that company's Specramast LC (lactating cow formula) product. It is resistant to hydrolysis by beta-lactamase, and has activity against both Gram-positive and Gram-negative bacteria. E. coli strains resistant to ceftiofur have been reported. The metabolite desfurolyceftiofur also has antibiotic activity, consequently the two compounds are measured together to monitor for antibiotic activity in the milk.
Ceftiofur is a semisynthetic, beta-lactamase-stable, broad-spectrum, third-generation cephalosporin with antibacterial activity. Ceftiofur binds to and inactivates penicillin-binding proteins (PBPs) located on the inner membrane of the bacterial cell wall. PBPs are enzymes involved in the terminal stages of assembling the bacterial cell wall and in reshaping the cell wall during growth and division. Inactivation of PBPs interferes with the cross-linkage of peptidoglycan chains necessary for bacterial cell wall strength and rigidity. This results in the weakening of the bacterial cell wall and causes cell lysis. See also: Ceftiofur Sodium (active moiety of); Ceftiofur Hydrochloride (has salt form); Ceftiofur Crystalline Free Acid (annotation moved to). Drug Indication PigsTreatment of bacterial respiratory disease associated with Actinobacillus pleuropneumoniae, Pasteurella multocida, Haemophilus parasuis and Streptococcus suis. Treatment of septicaemia, polyarthritis or polyserositis associated with Streptococcus suis infection. CattleTreatment of acute interdigital necrobacillosis in cattle also known as Panaritium or foot rot. Treatment of acute post-partum (puerperal) metritis in cattle, in cases where treatment with another antimicrobial has failed. Mechanism of Action Ceftiofur sodium is a third generation broad-spectrum cephalosporin, formulated as an intramuscular injection, which is used to treat respiratory diseases in swine, ruminants and horses. The thioester bond on ceftiofur is rapidly cleaved to give desfuroylceftiofur which is further metabolized to a disulfide dimer and various desfuroylceftiofur-protein and amino acid conjugates. Cephalosporins ... bind to penicillin-binding proteins located beneath the cell wall and thereby interfere with the action of transpeptidase and other cell-wall enzymes. A residual antibacterial effect is also evident with the cephalosporins. /Cephalosporins/ |
| 分子式 |
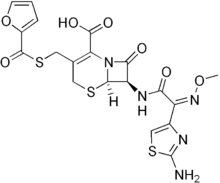
C19H17N5O7S3
|
|---|---|
| 分子量 |
523.55
|
| 精确质量 |
523.028
|
| CAS号 |
80370-57-6
|
| 相关CAS号 |
Ceftiofur hydrochloride;103980-44-5;Ceftiofur sodium;104010-37-9
|
| PubChem CID |
6328657
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.8±0.1 g/cm3
|
| 折射率 |
1.820
|
| LogP |
2.05
|
| tPSA |
256.26
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
13
|
| 可旋转键数目(RBC) |
9
|
| 重原子数目 |
34
|
| 分子复杂度/Complexity |
945
|
| 定义原子立体中心数目 |
2
|
| SMILES |
C(C1=C(CSC(C2OC=CC=2)=O)CS[C@@H]2[C@@H](C(N12)=O)NC(=O)/C(/C1N=C(N)SC=1)=N\OC)(=O)O
|
| InChi Key |
ZBHXIWJRIFEVQY-IHMPYVIRSA-N
|
| InChi Code |
InChI=1S/C19H17N5O7S3/c1-30-23-11(9-7-34-19(20)21-9)14(25)22-12-15(26)24-13(17(27)28)8(5-32-16(12)24)6-33-18(29)10-3-2-4-31-10/h2-4,7,12,16H,5-6H2,1H3,(H2,20,21)(H,22,25)(H,27,28)/b23-11-/t12-,16-/m1/s1
|
| 化学名 |
(6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-(furan-2-carbonylsulfanylmethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month 注意: 本产品在运输和储存过程中需避光。 |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~191.00 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.5 mg/mL (4.78 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入到400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (4.78 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.5 mg/mL (4.78 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.9100 mL | 9.5502 mL | 19.1004 mL | |
| 5 mM | 0.3820 mL | 1.9100 mL | 3.8201 mL | |
| 10 mM | 0.1910 mL | 0.9550 mL | 1.9100 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。