| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 500mg |
|
||
| 1g |
|
||
| 5g |
|
||
| Other Sizes |
|
| 靶点 |
Bacterial cell wall synthesis; penicillin binding proteins (PBPs); cephalosporin antibiotic
|
|---|---|
| 体外研究 (In Vitro) |
头孢氨苄 (10 μg/mL) 可使一种称为青霉素结合蛋白 (PBP) 的酶失活,该酶会干扰聚合物肽聚糖 (PG) 的合成 [1]。头孢氨苄可抑制多种革兰氏阳性和革兰氏阴性微生物,对雷氏变形杆菌的 MIC 值分别为 2、2、2、2、4、4.4 和 5.7 μg/mL [2]。
|
| 体内研究 (In Vivo) |
头孢氨苄(0-50 mg/kg;口服;3.5 小时)对感染微生物的雄性 Swiss-Webster 小鼠表现出抗菌作用 [2]。
|
| 酶活实验 |
青霉素和相关的β -内酰胺构成了我们最古老和最广泛使用的抗生素疗法之一。人们早就知道,这些药物的靶点是一种叫做青霉素结合蛋白(PBPs)的酶,这种酶负责构建细菌细胞壁。研究了靶抑制的下游后果,以及它们如何导致这些重要药物的致命作用,我们证明了β -内酰胺不仅仅是像通常认为的那样抑制PBPs。相反,它们诱导目标生物合成机制发生毒性故障,涉及细胞壁合成和降解的无效循环,从而耗尽细胞资源并增强其杀伤活性。这种作用模式的表征还揭示了细胞壁基质中裂解键的酶的质量控制功能。因此,这些结果提供了对细胞壁组装机制的深入了解,并建议如何最好地干预未来抗生素开发的过程。[1]
|
| 动物实验 |
Animal/Disease Models: Bacterially infected male Swiss-Webster mice [2]
Doses: 0-50 mg/kg Route of Administration: po (po (oral gavage)) 3.5 hrs (hrs (hours)) Experimental Results: Against Streptococcus pyogenes, Streptococcus pneumoniae, Staphylococcus aureus and several Antimicrobial activity against Gram-negative bacteria in mice. Cefadroxil is a new semisynthetic cephalosporin with a broad antibacterial spectrum and a high chemotherapeutic potential when administered orally. The inhibitory activity of this compound was similar to that of cephalexin and cephradine when tested against 602 clinical isolates on Mueller-Hinton medium. In the oral treatment of experimental infections of mice, cefadroxil was more effective than cephalexin against Streptococcus pyogenes, and comparably effective against Streptococcus pneumoniae, Staphylococcus aureus, and several gram-negative species. Administered orally to mice, at doses ranging from 25 to 100 mg/kg, cefadroxil attained peak concentrations in the blood similar to those of cephalexin. At a dose of 200 mg/kg, however, higher peak levels were noted with cefadroxil than with cephalexin. In regard to other properties which were investigated, the behavior of cefadroxil compared favorably to that of cephalexin.[2] |
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
Well absorbed from the upper gastrointestinal tract with nearly 100% oral bioavailability. Cephalexin is not absorbed in the stomach but is absorbed in the upper intestine. Patients taking 250mg of cephalexin reach a maximum plasma concentration of 7.7mcg/mL and patients taking 500mg reach 12.3mcg/mL. Cephalexin is over 90% excreted in the urine after 6 hours by glomerular filtration and tubular secretion with a mean urinary recovery of 99.3%. Cephalexin is unchanged in the urine. 5.2-5.8L. Clearance from one subject was 376mL/min. LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY... MORE THAN 90%...IS EXCRETED UNALTERED IN URINE WITHIN 6 HR, PRIMARILY BY RENAL TUBULAR SECRETION. ...THERAPEUTICALLY EFFECTIVE CONCN ARE STILL ACHIEVED IN URINE OF PT WITH DECR RENAL FUNCTION. CEPHALEXIN...IS WELL ABSORBED FROM GI TRACT. PEAK PLASMA CONCN, REACHED @ ABOUT 1 HR AFTER INGESTION OF DRUG, ARE APPROX 9 & 18 UG/ML AFTER ORAL DOSES OF 250 & 500 MG, RESPECTIVELY. INGESTION OF FOOD MAY DELAY ABSORPTION. CEPHALEXIN IS ALSO EXCRETED INTO BILE. BOTH ABSORPTION & EXCRETION OF CEPHALEXIN ARE IMPAIRED IN NEW-BORN INFANTS, WHERE 24-HR URINARY RECOVERY OF ANTIBIOTIC ACCOUNTED FOR 5-66% OF DAILY ORAL DOSE. For more Absorption, Distribution and Excretion (Complete) data for CEPHALEXIN (14 total), please visit the HSDB record page. Metabolism / Metabolites Cephalexin is not metabolized in the body. Biological Half-Life The half life of cephalexin is 49.5 minutes in a fasted state and 76.5 minutes with food though these times were not significantly different in the study. LESS THAN 10 TO 15%...IS BOUND TO PLASMA PROTEIN, & PLASMA DRUG CONCN FALL RAPIDLY, T/2 OF CEPHALEXIN NORMALLY BEING ABOUT 40 MIN. /IN RATS/ RATIOS OF BONE TO SERUM CONCN AVG...1:9 FOR CEPHALEXIN DURING 0.25-4 HR AFTER /ORAL/ DOSING. DESPITE DIFFERENCES IN CONCN; T/2 IN BONE & SERUM WERE SIMILAR. PEAK TIME, T/2 OF ELIMINATION, T/2 OF ABSORPTION, & VOL OF DISTRIBUTION WERE ALL SIMILAR FOLOWING ADMIN OF EITHER 1 OR 2 G OF CEPHALEXIN. The serum half-life of cephalexin is 0.5-1.2 hr in adults with normal renal function. The serum half-life of the drug is reported to be about 5 hr in neonates and 2.5 hr in children 3-12 mo of age. In one study, the serum half-life was 7.7 hr in adults with creatinine clearances of 9.2 ml/min and 13.9 hr in adults with creatinine clearances of 4 ml/min. |
| 毒性/毒理 (Toxicokinetics/TK) |
Interactions
PROBENECID IS EFFECTIVE IN SLOWING URINARY CLEARANCE & ENHANCING DURATION OF SYSTEMIC ANTIMICROBIAL ACTIVITY /OF CEPHALEXIN/. ...CEPHALOSPORINS...MAY BE AFFECTED BY CONCURRENT USE OF...SULFINPYRAZONE. DIMINISHED TUBULAR SECRETION...RESULT IN HIGHER & MORE SUSTAINED SERUM LEVELS & HENCE, INTENSIFICATION OF DRUG ACTIVITY. /CEPHALOSPORINS/ FUROSEMIDE MAY ENHANCE NEPHROTOXICITY OF CEPHALOSPORINS. /CEPHALOSPORINS/ Hypoprothrombinemia induced by large doses of salicylates and/or cephalosporins, and the gastrointestinal ulcerative or hemorrhagic potential of nonsteroidal anti-inflammatory drugs (NSAIDs), salicylates, or sulfinpyrazone may increase the risk of hemorrhage. /Cephalosporins/ For more Interactions (Complete) data for CEPHALEXIN (6 total), please visit the HSDB record page. Non-Human Toxicity Values LD50 MOUSE ORAL 1.6-4.5 G/KG /MONOHYDRATE/ LD50 MOUSE INTRAPERITONEAL 0.4-1.3 G/KG /MONOHYDRATE/ LD50 RAT ORAL GREATER THAN 5.0 G/KG /MONOHYDRATE/ LD50 RAT INTRAPERITONEAL GREATER THAN 3.7 G/KG /MONOHYDRATE/ |
| 参考文献 | |
| 其他信息 |
Therapeutic Uses
Cephalosporins /CEPHALEXIN/ HAS ANTIBACTERIAL SPECTRUM SIMILAR TO THAT OF PENICILLINS... AGAINST COCCI & GRAM-POSITIVE BACILLI, PENICILLIN G IS USUALLY MORE EFFECTIVE. ...MOST PENICILLINASES DO NOT AFFECT CEPHALEXIN... ...CEPHALOSPORINS ARE HIGHLY EFFECTIVE IN THERAPY OF VARIETY OF MILD-TO-SEVERE INFECTIONS DUE TO BOTH GRAM-POSITIVE & GRAM-NEGATIVE MICROORGANISMS. /CEPHALOSPORINS/ ...CEPHALOSPORIN IS...DRUG OF 1ST CHOICE...FOR KLEBSIELLA INFECTIONS. ... THEY ARE...VALUABLE SECONDARY AGENTS, & THEY FREQUENTLY APPEAR AS ALTERNATIVE CHOICES TO PENICILLIN. /CEPHALOSPORINS/ For more Therapeutic Uses (Complete) data for CEPHALEXIN (9 total), please visit the HSDB record page. Drug Warnings PHYSICIAN MUST ALTER EITHER DRUG DOSAGE OR INTERVAL BETWEEN DOSES WHEN RENAL FUNCTION IS IMPAIRED. CEPHALOSPORINS SHOULD NOT BE USED TO TREAT BACTERIAL MENINGITIS. THIS IS TRUE FOR ALL CAUSATIVE MICROORGANISMS. ...PENETRATION OF CEPHALOSPORINS INTO CSF IS POOR. /CEPHALOSPORINS/ INFECTIONS DUE TO ENTEROCOCCI ARE USUALLY UNAFFECTED BY THESE CMPD... ENTEROCOCCAL ENDOCARDITIS CANNOT BE CURED WITH CEPHALOSPORIN EVEN WHEN IT IS GIVEN CONCURRENTLY WITH GENTAMICIN OR STREPTOMYCIN. /CEPHALOSPORINS/ ENTEROBACTER (AEROBACTER) INFECTIONS ARE, AS A RULE, RESISTANT TO THESE CMPD. /CEPHALOSPORINS/ For more Drug Warnings (Complete) data for CEPHALEXIN (20 total), please visit the HSDB record page. Pharmacodynamics Cephalexin (also called Cefalexin) is a first generation cephalosporin antibiotic. It is one of the most widely prescribed antibiotics, often used for the treatment of superficial infections that result as complications of minor wounds or lacerations. It is effective against most gram-positive bacteria through its inihibition of the cross linking reaction between N-acetyl muramicacid and N-acetylglucosamine in the cell wall, leading to cell lysis. |
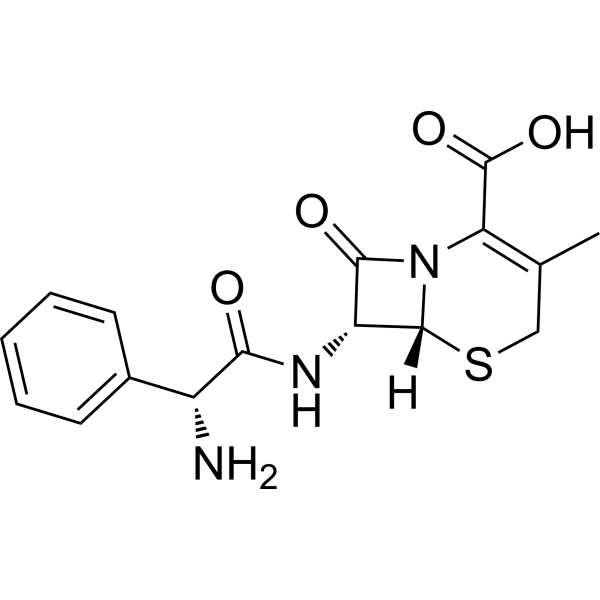
| 分子式 |
C16H17N3O4S
|
|---|---|
| 分子量 |
347.89
|
| 精确质量 |
347.093
|
| 元素分析 |
C, 55.32; H, 4.93; N, 12.10; O, 18.42; S, 9.23
|
| CAS号 |
15686-71-2
|
| 相关CAS号 |
Cephalexin hydrochloride;59695-59-9;Cephalexin monohydrate;23325-78-2;Cephalexin hydrochloride monohydrate;105879-42-3;Cephalexin (lysine);53950-14-4;Cephalexin-d5;2101505-56-8;
15686-71-2 (free); 38932-40-0 (sodium)
|
| PubChem CID |
27447
|
| 外观&性状 |
White to light yellow solid powder
|
| 密度 |
1.5±0.1 g/cm3
|
| 沸点 |
727.4±60.0 °C at 760 mmHg
|
| 熔点 |
196-198°C
|
| 闪点 |
393.7±32.9 °C
|
| 蒸汽压 |
0.0±2.5 mmHg at 25°C
|
| 折射率 |
1.700
|
| LogP |
0.65
|
| tPSA |
138.03
|
| 氢键供体(HBD)数目 |
3
|
| 氢键受体(HBA)数目 |
6
|
| 可旋转键数目(RBC) |
4
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
600
|
| 定义原子立体中心数目 |
3
|
| SMILES |
CC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)[C@@H](C3=CC=CC=C3)N)SC1)C(=O)O
|
| InChi Key |
ZAIPMKNFIOOWCQ-UEKVPHQBSA-N
|
| InChi Code |
InChI=1S/C16H17N3O4S/c1-8-7-24-15-11(14(21)19(15)12(8)16(22)23)18-13(20)10(17)9-5-3-2-4-6-9/h2-6,10-11,15H,7,17H2,1H3,(H,18,20)(H,22,23)/t10-,11-,15-/m1/s1
|
| 化学名 |
(6R,7R)-7-[[(2R)-2-amino-2-phenylacetyl]amino]-3-methyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
|
| 别名 |
Cephacillin; Cefalexin; Keflex; Cepexin; Carnosporin
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
H2O : ~10 mg/mL (~28.79 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 25 mg/mL (71.97 mM) in PBS (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液; 通过加热和超声助溶。
请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.8745 mL | 14.3724 mL | 28.7447 mL | |
| 5 mM | 0.5749 mL | 2.8745 mL | 5.7489 mL | |
| 10 mM | 0.2874 mL | 1.4372 mL | 2.8745 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。