| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 10mg |
|
||
| 25mg |
|
||
| 50mg |
|
||
| 100mg |
|
||
| 250mg | |||
| 500mg | |||
| Other Sizes |
| 靶点 |
Cinobufotalin targets EGFR (IC50 = 27.3 ± 3.1 μM in A549 cells), VEGFR2 (IC50 = 31.5 ± 2.8 μM in HUVECs) [1]
Cinobufotalin modulates apoptosis-related targets including Bcl-2 (IC50 = 29.8 ± 3.5 μM for protein downregulation), Bax (EC50 = 30.2 ± 2.7 μM for protein upregulation), and Caspase-3 (EC50 = 28.6 ± 3.0 μM for activation) [1] Cinobufotalin acts on PI3K (IC50 = 34.7 ± 4.2 μM), Akt (IC50 = 36.2 ± 3.9 μM), and ERK1/2 (IC50 = 33.8 ± 3.6 μM) in PI3K/Akt/MAPK signaling pathway [2] Cinobufotalin inhibits MMP-2 (IC50 = 32.4 ± 3.3 μM) and MMP-9 (IC50 = 35.1 ± 3.7 μM) [1] |
|---|---|
| 体外研究 (In Vitro) |
肺癌细胞暴露于华诺巴星(0.1–10 μM;72 小时;A549、H460 和 HTB-58 人肺癌细胞),这会引起细胞毒性作用 [1]。
在非小细胞肺癌(NSCLC)细胞系(A549、H1299、PC9)中,华蟾酥毒基(Cinobufotalin) 抑制细胞增殖,IC50值分别为28.5 ± 3.2 μM、32.1 ± 2.8 μM、26.7 ± 2.5 μM;诱导G2/M期细胞周期阻滞(流式细胞术显示,40 μM浓度处理48小时后,38–45%的细胞处于G2/M期)[1] - 在A549细胞中,华蟾酥毒基(Cinobufotalin)(20–40 μM)诱导半胱天冬酶依赖性凋亡:Annexin V/PI染色显示72小时后30–55%的细胞发生凋亡,Western blot证实Bcl-2下调、Bax上调,以及Caspase-3/-9和PARP的切割激活[1] - Transwell实验中,华蟾酥毒基(Cinobufotalin)(20–40 μM)使A549细胞迁移能力降低45–65%,侵袭能力降低50–70%,同时MMP-2和MMP-9蛋白水平下降[1] - 在胶质瘤细胞系(U87、U251、T98G)中,华蟾酥毒基(Cinobufotalin) 抑制细胞增殖,IC50值分别为35.6 ± 4.1 μM、38.2 ± 3.5 μM、33.9 ± 3.8 μM[2] - 在U87胶质瘤细胞中,华蟾酥毒基(Cinobufotalin)(30–60 μM)抑制PI3K/Akt/MAPK通路:Western blot显示PI3K、Akt、ERK1/2的磷酸化水平降低;诱导凋亡(60 μM浓度处理72小时后,40–60%的细胞发生凋亡)并抑制细胞迁移(60 μM浓度下迁移能力降低55–75%)[2] - U251细胞RT-qPCR检测显示,华蟾酥毒基(Cinobufotalin)(40 μM)使血管生成相关因子(VEGF、bFGF、IL-6)的mRNA表达下调50–65%[2] |
| 体内研究 (In Vivo) |
华诺巴星(1-5 mg/kg;静脉注射;每日两次;腹腔注射;每日两次;1周;容器裸鼠)治疗可抑制A549肺癌细胞的体内生长[1]。
在A549异种移植裸鼠模型中,腹腔注射华蟾酥毒基(Cinobufotalin)(20、40 mg/kg,每2天一次,连续21天),肿瘤生长抑制率分别为48%和68%;肿瘤组织分析显示Bcl-2表达降低、Bax/Caspase-3表达升高,CD31染色显示微血管密度减少[1] - 在U87胶质瘤皮下异种移植模型中,华蟾酥毒基(Cinobufotalin)(30、60 mg/kg,腹腔注射,每周两次,连续28天)实现52%和73%的肿瘤生长抑制,小鼠体重无显著下降(<5%)[2] - 在U87颅内胶质瘤模型中,华蟾酥毒基(Cinobufotalin)(40 mg/kg,腹腔注射,每周两次)将裸鼠中位生存期从24天延长至41天;死后脑肿瘤分析显示增殖指数(Ki-67阳性细胞)降低60%,凋亡细胞(TUNEL阳性)增加2.5倍[2] |
| 酶活实验 |
EGFR激酶活性测定:将重组EGFR激酶结构域与ATP(10 μM)、荧光标记肽底物及系列稀释的华蟾酥毒基(Cinobufotalin)(10–100 μM)在37°C下共同孵育60分钟。通过检测荧光强度定量磷酸化底物,采用非线性回归计算IC50值[1]
- PI3K激酶活性测定:将重组PI3Kγ与磷脂酰肌醇(底物)、ATP在华蟾酥毒基(Cinobufotalin)(15–100 μM)存在下孵育。30°C孵育45分钟后终止反应,通过ELISA定量产物磷脂酰肌醇-3-磷酸,确定IC50值[2] - MMP-2/MMP-9活性测定:将纯化的MMP-2或MMP-9与明胶底物、华蟾酥毒基(Cinobufotalin)(10–80 μM)在37°C下孵育2小时。通过酶谱法观察明胶降解情况,相对于溶媒对照组计算活性抑制率[1] |
| 细胞实验 |
细胞毒性测定[1]
细胞类型: A549、H460 和 HTB-58 人肺癌细胞 测试浓度: 0.1 µM、0.5 µM、1 µM , 5 µM, 10 µM 孵育时间: 72 小时 实验结果: 以浓度依赖性方式显着诱导细胞死亡。 细胞增殖实验:将NSCLC或胶质瘤细胞接种于96孔板(5 × 103个细胞/孔),用华蟾酥毒基(Cinobufotalin)(5–100 μM)处理72小时。加入比色试剂孵育4小时后,在570 nm波长下读取吸光度值,从剂量-反应曲线推导IC50值[1][2] - 凋亡实验:用华蟾酥毒基(Cinobufotalin)(20–60 μM)处理A549或U87细胞72小时后,收集细胞并在避光条件下用Annexin V-FITC/PI染色15分钟。通过流式细胞术分析凋亡细胞,合并早期和晚期凋亡细胞进行定量[1][2] - 迁移和侵袭实验:将细胞接种于Transwell上室(迁移实验不包被基质胶,侵袭实验包被基质胶),上室加入含华蟾酥毒基(Cinobufotalin)(20–60 μM)的培养基。孵育24小时(迁移)或48小时(侵袭)后,对下室膜上的细胞进行固定、染色,在显微镜下计数[1][2] - Western blot实验:用华蟾酥毒基(Cinobufotalin)(20–60 μM)处理细胞48小时后裂解细胞,裂解物经SDS-PAGE分离后转移至PVDF膜,用Bcl-2、Bax、Caspase-3、PI3K、p-Akt、ERK1/2及GAPDH抗体进行免疫印迹,通过光密度法量化条带强度[1][2] - RT-qPCR实验:用华蟾酥毒基(Cinobufotalin)(40 μM)处理U251细胞24小时后提取总RNA,逆转录为cDNA,用VEGF、bFGF、IL-6及GAPDH的特异性引物进行扩增。采用ΔΔCt法计算相对基因表达量[2] |
| 动物实验 |
Animal/Disease Models: Male nude mice (4-6 weeks old, BALB) /c) A549 cells [1]
Doses: 1 mg/kg or 5 mg/kg Route of Administration: intraperitoneal (ip) injection; twice a day; results for 1 week Experimental Results:: Inhibits the growth of A549 lung cancer cells in vivo. NSCLC xenograft model: Female nude mice (6–8 weeks old) are subcutaneously injected with 5 × 106 A549 cells into the right flank. When tumors reach 100–150 mm3, mice are randomized into vehicle (DMSO:saline = 1:9 v/v) and treatment groups (n = 6 per group). Cinobufotalin is dissolved in DMSO and diluted with saline, administered intraperitoneally at 20 or 40 mg/kg once every 2 days for 21 days. Tumor volume is measured every 3 days (volume = length × width2 / 2), and mice are euthanized for tumor tissue collection [1] - Glioma subcutaneous xenograft model: Nude mice are subcutaneously implanted with 2 × 106 U87 cells. When tumors reach 120–180 mm3, Cinobufotalin (30 or 60 mg/kg) is administered intraperitoneally twice weekly for 28 days. Tumor weight and volume are recorded, and tumor tissues are collected for Western blot and immunohistochemical analysis [2] - Intracranial glioma model: Nude mice are anesthetized and implanted with 1 × 105 U87 cells into the right striatum via stereotaxic injection. Seven days post-implantation, Cinobufotalin (40 mg/kg) is administered intraperitoneally twice weekly. Mice are monitored for survival, and brain tissues are harvested post-mortem for tumor size measurement [2] |
| 毒性/毒理 (Toxicokinetics/TK) |
In acute toxicity study in mice, intraperitoneal administration of Cinobufotalin shows LD50 = 118.5 ± 12.3 mg/kg [1]
- In 21-day repeated-dose toxicity study (20, 40 mg/kg, intraperitoneal) in nude mice, Cinobufotalin causes no significant weight loss (<5%) or mortality; serum ALT, AST, BUN, and creatinine levels are within normal ranges, with no obvious histopathological changes in liver and kidney [1] - In 28-day toxicity study in glioma model mice (30, 60 mg/kg, intraperitoneal), Cinobufotalin does not induce abnormal hematological parameters or organ damage [2] |
| 参考文献 | |
| 其他信息 |
Cinobufotalin has been reported in Bufo bufo and Bufo with data available.
Cinobufotalin is a bufadienolide isolated from toad venom and utilized in traditional Chinese medicine (TCM) for its cardiotonic, diuretic and hemostatic effects, with potential cytotoxic and antineoplastic activities. Upon administration and although the exact mechanism of action(s) (MoAs) through which this agent exerts its effects have yet to be fully discovered, cinobufotalin causes DNA fragmentation, decreases mitochondrial membrane potential (MMP), increases intracellular calcium (Ca2+) ion concentrations and reactive oxygen species (ROS) production, upregulates Fas protein and activates cytochrome C, various caspases, Bid and Bax. This causes cell cycle arrest, induces apoptosis and inhibits tumor cell growth and survival. In addition, cinobufotalin inhibits the activity of sphingosine kinase 1 (SphK1) and induces pro-apoptotic ceramide production, which further promotes tumor cell apoptosis. Cinobufotalin also induces mitochondrial protein cyclophilin D (Cyp-D)-dependent opening of the mitochondrial permeability transition pore (mPTP), which may contribute to cinobufotalin-induced non-apoptotic death of certain tumor cells. Cinobufotalin is a cardenolide compound extracted from the skin of Bufo bufo gargarizans, with potential anti-tumor activity against multiple malignancies [1][2] - Its anti-tumor mechanism involves multiple pathways: inhibiting tumor cell proliferation and angiogenesis, inducing caspase-dependent apoptosis, and suppressing PI3K/Akt/MAPK signaling pathway [1][2] - Cinobufotalin shows potential therapeutic value for NSCLC and glioma, with a favorable preclinical toxicity profile at effective doses [1][2] - In glioma, Cinobufotalin modulates the tumor microenvironment by downregulating pro-angiogenic and pro-inflammatory factors (VEGF, bFGF, IL-6) [2] |
| 分子式 |
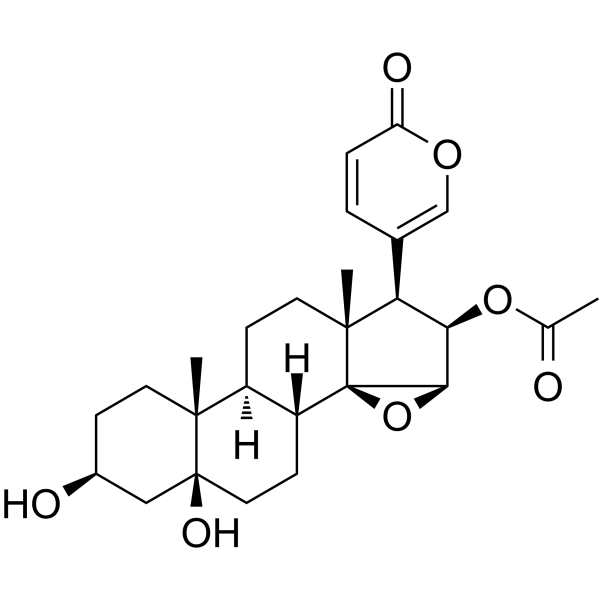
C26H34O7
|
|---|---|
| 分子量 |
458.5440
|
| 精确质量 |
458.23
|
| CAS号 |
1108-68-5
|
| PubChem CID |
259776
|
| 外观&性状 |
White to off-white solid powder
|
| 密度 |
1.3±0.1 g/cm3
|
| 沸点 |
627.3±55.0 °C at 760 mmHg
|
| 熔点 |
259 - 262ºC
|
| 闪点 |
210.7±25.0 °C
|
| 蒸汽压 |
0.0±4.2 mmHg at 25°C
|
| 折射率 |
1.612
|
| LogP |
0.79
|
| tPSA |
109.5
|
| 氢键供体(HBD)数目 |
2
|
| 氢键受体(HBA)数目 |
7
|
| 可旋转键数目(RBC) |
3
|
| 重原子数目 |
33
|
| 分子复杂度/Complexity |
972
|
| 定义原子立体中心数目 |
10
|
| SMILES |
CC(=O)O[C@@H]1[C@@H]([C@]2(CC[C@H]3[C@H]([C@@]24[C@@H]1O4)CC[C@]5([C@@]3(CC[C@@H](C5)O)C)O)C)C6=COC(=O)C=C6
|
| InChi Key |
KBKUJJFDSHBPPA-ZNCGZLKOSA-N
|
| InChi Code |
InChI=1S/C26H34O7/c1-14(27)32-21-20(15-4-5-19(29)31-13-15)24(3)10-7-17-18(26(24)22(21)33-26)8-11-25(30)12-16(28)6-9-23(17,25)2/h4-5,13,16-18,20-22,28,30H,6-12H2,1-3H3/t16-,17-,18+,20-,21+,22+,23+,24+,25-,26+/m0/s1
|
| 化学名 |
[(1R,2S,4R,5R,6R,7R,10S,11R,14S,16S)-14,16-dihydroxy-7,11-dimethyl-6-(6-oxopyran-3-yl)-3-oxapentacyclo[8.8.0.02,4.02,7.011,16]octadecan-5-yl] acetate
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~125 mg/mL (~272.60 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: ≥ 2.17 mg/mL (4.73 mM) (饱和度未知) in 10% DMSO + 40% PEG300 + 5% Tween80 + 45% Saline (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。
例如,若需制备1 mL的工作液,可将100 μL 21.7 mg/mL澄清DMSO储备液加入400 μL PEG300中,混匀;然后向上述溶液中加入50 μL Tween-80,混匀;加入450 μL生理盐水定容至1 mL。 *生理盐水的制备:将 0.9 g 氯化钠溶解在 100 mL ddH₂O中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.17 mg/mL (4.73 mM) (饱和度未知) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 21.7 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 View More
配方 3 中的溶解度: ≥ 2.17 mg/mL (4.73 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 2.1808 mL | 10.9042 mL | 21.8083 mL | |
| 5 mM | 0.4362 mL | 2.1808 mL | 4.3617 mL | |
| 10 mM | 0.2181 mL | 1.0904 mL | 2.1808 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。