| 规格 | 价格 | 库存 | 数量 |
|---|---|---|---|
| 50mg |
|
||
| 100mg |
|
||
| 250mg |
|
||
| 500mg |
|
||
| Other Sizes |
|
| 药代性质 (ADME/PK) |
Absorption, Distribution and Excretion
The single- and multiple-dose pharmacokinetics of citalopram are linear and dose-proportional in a dose range of 10 to 40 mg/day. Biotransformation of citalopram is mainly hepatic, with a mean terminal half-life of about 35 hours. With once daily dosing, steady state plasma concentrations are achieved within approximately one week. At steady state, the extent of accumulation of citalopram in plasma, based on the half-life, is expected to be 2.5 times the plasma concentrations observed after a single dose. Following a single oral dose (40 mg tablet) of citalopram, peak blood levels occur at about 4 hours. The absolute bioavailability of citalopram was about 80% relative to an intravenous dose, and absorption is not affected by food. Approximately 12 to 23% of an oral dose of citalopram is found unchanged in the urine, while 10% is found in feces. Following intravenous administrations of citalopram, the fraction of the drug recovered in the urine as citalopram and DCT was about 10% and 5%, respectively. The volume of distribution of citalopram is about 12 L/kg. The systemic clearance of citalopram was 330 mL/min, with approximately 20% of that due to renal clearance. Like other selective serotonin-reuptake inhibitors, citalopram is a highly lipophilic compound that appears to be rapidly and well absorbed from the GI tract following oral administration. Following a single 40-mg oral dose of citalopram as a tablet, the manufacturer states that peak plasma concentrations averaging approximately 44 ng/mL occur at about 4 hours. The absolute bioavailability of citalopram is approximately 80% relative to an IV dose. The oral tablets and solution of citalopram reportedly are bioequivalent. Food does not substantially affect the absorption of citalopram. Distribution of citalopram and its metabolites into human body tissues and fluids has not been fully characterized. However, limited pharmacokinetic data suggest that the drug, which is highly lipophilic, is widely distributed in body tissues. /MILK/ /The purpose of the study was/ to characterize milk/plasma (M/P) ratio and infant dose, for citalopram and demethylcitalopram, in breast-feeding women taking citalopram for the treatment of depression, and to determine the plasma concentration and effects of these drugs in their infants. Seven women (mean age 30.6 years) taking citalopram (median dose 0.36 mg/kg/day) and their infants (mean age 4.1 months) were studied. Citalopram and demethylcitalopram in plasma and milk were measured by high-performance liquid chromatography over a 24 hr dose interval. Infant exposure was estimated (two separate methods) as the product of milk production rate and drug concentration in milk, normalized to body weight and expressed as a percentage of the weight-adjusted maternal dose. Mean M/PAUC values of 1.8 (range 1.2-3) and 1.8 (range 1.0-2.5) were calculated for citalopram and demethylcitalopram, respectively. The mean maximum concentrations of citalopram and demethylcitalopram in milk were 154 (95% CI, 102-207) ug/L and 50 (23-77) ug/L. Depending on the method of calculation, mean infant exposure was 3.2 or 3.7% for citalopram and 1.2 or 1.4% for demethylcitalopram. Citalopram (2.0, 2.3 and 2.3 ug/L) was detected in three of the seven infants. Demethylcitalopram (2.2 and 2.2 ug/L was detected in plasma from two of the same infants. ... The mean combined dose of citalopram and demethylcitalopram (4.4-5.1% as citalopram equivalents) transmitted to infants via breast milk is below the 10% notional level of concern. ... For more Absorption, Distribution and Excretion (Complete) data for Citalopram (14 total), please visit the HSDB record page. Metabolism / Metabolites Citalopram is metabolized mainly in the liver via N-demethylation to its main metabolite, _demethylcitalopram_ by CYP2C19 and CYP3A4. Other metabolites include _didemethylcitalopram_ via CYP2D6 metabolism, _citalopram N-oxide_ and propionic acid derivative via monoamine oxidase enzymes A and B and aldehyde oxidase. Citalopram metabolites exert little pharmacologic activity in comparison to the parent drug and are not likely to contribute to the clinical effect of citalopram. In a 2002 study, 11 women took citalopram (20-40 mg/day) during pregnancy; 10 throughtout gestation and 1 starting at 20 weeks' gestation. The mean ratio of two metabolites was significantly higher during pregnancy than at 2 months postdelivery, indicating induction of the CYP2D6 isoenzyme. The trough plasma concentration of citalopram, desmethylcitalopram, and didesmethylcitalopram in the normal new borns were 64%, 66%, and 68% of the maternal concentrations, respectively. The antidepressant citalopram (CT), a selective serotonin uptake inhibitor, was given in its labelled form, 14(C)-CT, as a single oral dose in 50 mL aqueous solution (0.1 mmol/30 uCi/1.1 MBq) to four healthy male volunteers. Concentrations of radioactivity in whole blood and plasma were similar. ... The HPLC profile of urinary components showed that besides the known metabolites of citalopram, three glucuronides were present. The relative amounts of CT and its metabolites in urine collected for 7 days were: CT (26 %), N-demethyl-CT (DCT, 19%), N,N-didemethyl-CT (DDCT,9%), the N-oxide (7%), the quaternary ammonium glucuronide of CT (CT-GLN, 14%), the N-glucuronide of DDCT (DDCT-GLN, 6%), and the glucuronide of the acid metabolite (CT-acid-GLN, 12%) formed by N,N-dimethyl deamination of CT. CT-GLN was isolated using preparative chromatography and identified by LC-MS-MS and NMR. DDCT-GLN and CT-acid-GLN were identified by LC-MS. This study shows that protracted renal excretion represents the major route of elimination, with a small fraction voided with feces. A considerable portion of the urinary excreted dose consists of N-glucuronides of CT and DDCT together with the O-acyl glucuronide of CT-acid. This study was conducted to identify enzyme systems eventually catalyzing a local cerebral metab of citalopram. ... The metab of citalopram, of its enantiomers and demethylated metabolites was investigated in rat brain microsomes & in rat and human brain mitochondria. No cytochrome P-450 mediated transformation was observed in rat brain. ... In rat whole brain and in human frontal cortex, putamen, cerebellum & white matter of five brains monoamine oxidase activity was determined by the stereoselective measurement of the production of citalopram propionate. All substrates were metabolized by both forms of MAO, except in rat brain, where monoamine oxidase B activity could not be detected. Apparent Km and Vmax of S-citalopram biotransformation in human frontal cortex by monoamine oxidase B were found to be 266 uM & 6.0 pmol min/mg protein & by monoamine oxidase A 856 uM & 6.4 pmol min/mg protein, respectively. These Km values are in the same range as those for serotonin & dopamine metab by monoamine oxidases. ... Citalopram ... is N-demethylated to N-desmethylcitalopram partially by CYP2C19 & partially by CYP3A4 & N-desmethylcitalopram is further N-demethylated by CYP2D6 to the likewise inactive metabolite di-desmethylcitalopram. The two metabolites are not active. ... In vitro citalopram does not inhibit CYP or does so only very moderately. A number of studies in healthy subjects and patients have confirmed, that this also holds true in vivo. Thus no change in pharmacokinetics or only very small changes were observed when citalopram was given with CYP1A2 substrates (clozapine & therophylline), CYP2C9 (warfarin), CYP2C19 (imipramine & mephenytoin), CYP2D6 (sparteine, imipramine & amitriptyline) and CYP3A4 (carbamazepine & triazolam). ... For more Metabolism/Metabolites (Complete) data for Citalopram (7 total), please visit the HSDB record page. Citalopram has known human metabolites that include N-Desmethylcitalopram and Citalopram N-oxide. Citalopram is metabolized mainly in the liver via N-demethylation to its principle metabolite, demethylcitalopram. Other metabolites include didemethylcitalopram, citalopram N-oxide, and a deaminated propionic acid derivative. However, the predominant entity in plasma is unchanged citalopram. Cytochrome P450 (CYP) 3A4 and 2C19 isozymes appear to be principally involved in producing demethylcitalopram. Demethylcitalopram appears to be further N-demethylated by CYP2D6 to didemethylcitalopram. Citalopram metabolites possess little pharmacologic activity in comparison to their parent compound and do not likely contribute to the clinical effect of the drug. Route of Elimination: 12-23% of an oral dose of citalopram is recovered unchanged in the urine, while 10% of the dose is recovered in the feces. Half Life: 35 hours Biological Half-Life The mean terminal half-life of citalopram is about 35 hours. The antidepressant citalopram (CT), a selective serotonin uptake inhibitor, was given in its labelled form, 14(C)-CT, as a single oral dose in 50 mL aqueous solution (0.1 mmol/30 uCi/1.1 MBq) to four healthy male volunteers. Concentrations of radioactivity in whole blood and plasma were similar. The respective pharmacokinetic parameters were: ... half life = 90.2+/-22.5 and 79.5 +/- 14.9 hr respectively. ... The elimination half-life of citalopram averages approximately 35 hours in adults with normal renal and hepatic function. |
|---|---|
| 毒性/毒理 (Toxicokinetics/TK) |
Toxicity Summary
IDENTIFICATION AND USE: Citalopram is a solid. It is a serotonin uptake inhibitor, and second-generation antidepressive agent. Citalopram tablets are indicated for the treatment of depression. HUMAN STUDIES: Symptoms most often accompanying citalopram overdose, alone or in combination with other drugs and/or alcohol, included dizziness, sweating, nausea, vomiting, tremor, somnolence, and sinus tachycardia. In more rare cases, observed symptoms included amnesia, confusion, coma, convulsions, hyperventilation, cyanosis, rhabdomyolysis, and ECG changes (including QTc prolongation, nodal rhythm, ventricular arrhythmia, and very rare cases of torsade de pointes). Acute renal failure has been very rarely reported accompanying overdose. Five male infants exposed to citalopram (30 mg/day), paroxetine (10-40 mg/day) or fluoxetine (20 mg/day) during gestation exhibited withdrawal symptoms at or within a few days of birth and lasting up to 1 month. Symptoms included irritability, constant crying, shivering, increased tonus, eating and sleeping problems, and convulsions. Late gestational exposure to citalopram, may be associated with a neonatal toxicity syndrome with immediate onset at birth or soon after birth and sometimes may be mistaken for neonatal withdrawal syndrome. A 3860 g infant was delivered at 40 weeks gestation. The mother had been taking citalopram 20 mg/day until the day of delivery. Citalopram was not clastogenic in the in vitro chromosomal aberration assay in human lymphocytes. ANIMAL STUDIES: Citalopram was administered in the diet to mice and rats for 18 and 24 months, respectively. There was no evidence for carcinogenicity of citalopram in mice receiving up to 240 mg/kg/day. There was an increased incidence of small intestine carcinoma in rats receiving 8 or 24 mg/kg/day doses. The relevance of these findings to humans is unknown. Pathologic changes (degeneration/atrophy) were observed in the retinas of albino rats in the 2-year carcinogenicity study with citalopram. In a rabbit study, no adverse effects on embryo/fetal development were observed at doses of up to 16 mg/kg/day. When female rats were treated with citalopram (4.8, 12.8, or 32 mg/kg/day) from late gestation through weaning, increased offspring mortality during the first 4 days after birth and persistent offspring growth retardation were observed at the highest dose. In two rat embryo/fetal development studies, oral administration of citalopram (32, 56, or 112 mg/kg/day) to pregnant animals during the period of organogenesis resulted in decreased embryo/fetal growth and survival and an increased incidence of fetal abnormalities (including cardiovascular and skeletal defects) at the high dose. Citalopram was mutagenic in the in vitro bacterial reverse mutation assay (Ames test) in 2 of 5 bacterial strains (Salmonella TA98 and TA1537) in the absence of metabolic activation. It was clastogenic in the in vitro Chinese hamster lung cell assay for chromosomal aberrations in the presence and absence of metabolic activation. Citalopram was not mutagenic in the in vitro mammalian forward gene mutation assay (HPRT) in mouse lymphoma cells or in a coupled in vitro/in vivo unscheduled DNA synthesis (UDS) assay in rat liver. The antidepressant, antiobsessive-compulsive, and antibulimic actions of citalopram are presumed to be linked to its inhibition of CNS neuronal uptake of serotonin. Citalopram blocks the reuptake of serotonin at the serotonin reuptake pump of the neuronal membrane, enhancing the actions of serotonin on 5HT1A autoreceptors. SSRIs bind with significantly less affinity to histamine, acetylcholine, and norepinephrine receptors than tricyclic antidepressant drugs. Interactions The aim of this study was to investigate the drug-drug interaction between carvedilol and citalopram based on carvedilol metabolism in vitro and his pharmacokinetics (PKs) in vivo after the oral administration of the single drug and both drugs, and reveal citalopram effects on the PKs of carvedilol. Each rat was cannulated on the femoral vein, prior to being connected to BASi Culex ABC. Carvedilol was orally administrated in rats (3.57 mg/kg body weight (bw)) in the absence of citalopram or after a pre-treatment with multiple oral doses of citalopram (1.42 mg/kg b.w.). Plasma concentrations of carvedilol were determined using high-performance liquid chromatography-MS at the designated time points after drug administration, and the main PK parameters were calculated by noncompartmental analysis. In addition, effects of citalopram on the metabolic rate of carvedilol were investigated using rat-pooled liver microsome incubation systems. During co-administration, significant increases of the area under the plasma concentration-time curve as well as of the peak plasma concentration were observed. The rat-pooled liver microsome incubation experiment indicated that citalopram could decrease the metabolic rate of carvedilol. Citalopram co-administration led to a significant alteration of carvedilol's PK profile in rats; it also demonstrated, in vitro, these effects could be explained by the existence of a drug-drug interaction mediated by CYP2D6 inhibition. ... A case involving a fatality due to the combined ingestion of two different types of antidepressants /is presented/. A 41-year-old Caucasian male, with a history of depression and suicide attempts, was found deceased at home. Multiple containers of medication, /including/ citalopram (Cipramil) ... as well as a bottle of whiskey were present at the scene. The autopsy findings were unremarkable, but systematic toxicological analysis ... revealed citalopram (and metabolite) /among others/. ... A new liquid chromatographic separation /was developed/ ... and the results obtained for blood and urine, respectively, were /for/ desmethylcitalopram 0.42 ug/mL and 1.22 ug/mL; & /for/ citalopram 4.47 ug/mL and 19.7 ug/mL /among others/. The cause of death was attributed to the synergistic toxicity of moclobemide and citalopram ... /which/ can produce a potentially lethal hyperserotoninergic state ... The purpose of this study was to evaluate the influence of tobacco smoke on the pharmacokinetics of citalopram (CIT) and desmethylcitalopram (DCIT) and its enantiomers on an animal model. High performance liquid chromatography (HPLC) with a diode array detector (DAD) was used for the identification and quantification of the studied compounds. The HPLC quantification of racemic mixtures of CIT was performed on a C18 column. The limits of detection (LOD) and quantification (LOQ) were: 7 and 10 ng/mL respectively. HPLC separation of citalopram enantiomers (S- and R-CIT) was performed on a Chirobiotic V column. The limits of detection (LOD) and quantification (LOQ) were: 6 and 15 ng/mL for R- and S-CIT respectively. The experiment was carried out on male Wistar rats. The rats were exposed to tobacco smoke for five days (6 hours per day). After the exposure, citalopram was administered in a dose of 10 mg/kg intragastrically. In the control group (non-exposed animals), citalopram was administered in the same way and at an equal dose. The blood of the animals was collected at nine time points. It was found that tobacco smoke exposure inhibits the biotransformation of citalopram. The half-life of the racemic mixture of citalopram after intragastric administration was increased by about 287%. Changes in the pharmacokinetic parameters of S-citalopram (active isomer) show a similar tendency to those of the racemic mixture. The pharmacokinetics of R-citalopram showed no statistically important differences after tobacco smoke exposure. Alterations in the pharmacological parameters of desmethylcitalopram presented an opposite trend to the parent drug. After exposure to tobacco smoke, the induction of metabolism of this compound was observed. Use of selective serotonin-reuptake inhibitors (SSRIs) such as citalopram concurrently or in close succession with other drugs that affect serotonergic neurotransmission may result in potentially life-threatening serotonin syndrome. Manifestations of serotonin syndrome may include mental status changes (e.g., agitation, hallucinations, delirium, coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or GI symptoms (e.g., nausea, vomiting, diarrhea). The precise mechanism of serotonin syndrome is not fully understood; however, it appears to result from excessive serotonergic activity in the CNS, probably mediated by activation of serotonin 5-HT1A receptors. The possible involvement of dopamine and 5-HT2 receptors also has been suggested, although their roles remain unclear. For more Interactions (Complete) data for Citalopram (34 total), please visit the HSDB record page. |
| 参考文献 |
|
| 其他信息 |
Therapeutic Uses
Serotonin Uptake Inhibitors; Antidepressive Agents, Second-Generation /CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Citalopram is included in the database. Citalopram tablets are indicated for the treatment of depression. /Included in US product label/ Citalopram has been used in the treatment of obsessive-compulsive disorder. /NOT included in US product label/ For more Therapeutic Uses (Complete) data for Citalopram (16 total), please visit the HSDB record page. Drug Warnings /BOXED WARNING/ SUICIDALITY AND ANTIDEPRESSANT DRUGS. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of citalopram or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Citalopram is not approved for use in pediatric patients. It is recommended that citalopram should not be used in patients with congenital long QT syndrome, bradycardia, hypokalemia or hypomagnesemia, recent acute myocardial infarction, or uncompensated heart failure. Citalopram should also not be used in patients who are taking other drugs that prolong the QTc interval. Such drugs include Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone). The citalopram dose should be limited in certain populations. The maximum dose should be limited ... in patients who are CYP2C19 poor metabolizers or those patients who may be taking concomitant cimetidine or another CYP2C19 inhibitor, since higher citalopram exposures would be expected. The maximum dose should also be limited ... in patients with hepatic impairment and in patients who are greater than 60 years of age because of expected higher exposures. The development of a potentially life-threatening serotonin syndrome has been reported with selective norepinephrine-reuptake inhibitors (SNRIs) and serotonin-reuptake inhibitors (SSRIs), including citalopram tablets, alone but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John's Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome. For more Drug Warnings (Complete) data for Citalopram (49 total), please visit the HSDB record page. Pharmacodynamics Citalopram belongs to a class of antidepressants known as selective serotonin reuptake inhibitors (SSRIs). It has been found to relieve or manage symptoms of depression, anxiety, eating disorders and obsessive-compulsive disorder among other mood disorders. The antidepressant, anti-anxiety, and other actions of citalopram are linked to its inhibition of CNS central uptake of serotonin. Serotonergic abnormalities have been reported in patients with mood disorders. Behavioral and neuropsychological effects of serotonin include the regulation of mood, perception, reward, anger, aggression, appetite, memory, sexuality, and attention, as examples. The onset of action for depression is approximately 1 to 4 weeks. The complete response may take 8-12 weeks after initiation of citalopram. In vitro studies demonstrate that citalopram is a strong and selective inhibitor of neuronal serotonin reuptake and has weak effects on norepinephrine and dopamine central reuptake. The chronic administration of citalopram has been shown to downregulate central norepinephrine receptors, similar to other drugs effective in the treatment of major depressive disorder. Citalopram does not inhibit monoamine oxidase. |
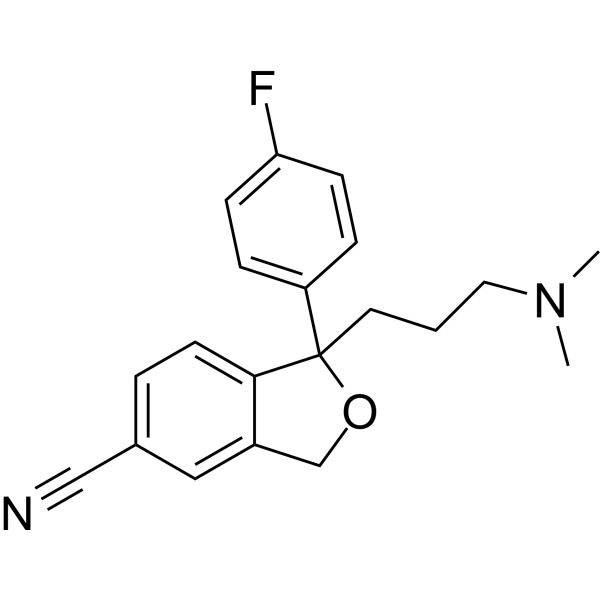
| 分子式 |
C20H21FN2O
|
|---|---|
| 分子量 |
324.39
|
| 精确质量 |
324.163
|
| CAS号 |
59729-33-8
|
| 相关CAS号 |
Citalopram-d4 hydrobromide;1219803-58-3;Citalopram-d6;1190003-26-9;Citalopram hydrobromide;59729-32-7;Citalopram-d6 oxalate;1246819-94-2
|
| PubChem CID |
2771
|
| 外观&性状 |
Colorless to light yellow ointment
|
| 密度 |
1.2±0.1 g/cm3
|
| 沸点 |
428.3±45.0 °C at 760 mmHg
|
| 熔点 |
180 - 187ºC
|
| 闪点 |
212.8±28.7 °C
|
| 蒸汽压 |
0.0±1.0 mmHg at 25°C
|
| 折射率 |
1.591
|
| LogP |
2.51
|
| tPSA |
36.26
|
| 氢键供体(HBD)数目 |
0
|
| 氢键受体(HBA)数目 |
4
|
| 可旋转键数目(RBC) |
5
|
| 重原子数目 |
24
|
| 分子复杂度/Complexity |
466
|
| 定义原子立体中心数目 |
0
|
| SMILES |
CN(C)CCCC1(C2=CC=C(C=C2)F)C3=C(C=C(C=C3)C#N)CO1
|
| InChi Key |
WSEQXVZVJXJVFP-UHFFFAOYSA-N
|
| InChi Code |
InChI=1S/C20H21FN2O/c1-23(2)11-3-10-20(17-5-7-18(21)8-6-17)19-9-4-15(13-22)12-16(19)14-24-20/h4-9,12H,3,10-11,14H2,1-2H3
|
| 化学名 |
1-[3-(dimethylamino)propyl]-1-(4-fluorophenyl)-3H-2-benzofuran-5-carbonitrile
|
| 别名 |
Lu-10-171 Lu10-171; Lu 10-171; Citalopram
|
| HS Tariff Code |
2934.99.9001
|
| 存储方式 |
Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month |
| 运输条件 |
Room temperature (This product is stable at ambient temperature for a few days during ordinary shipping and time spent in Customs)
|
| 溶解度 (体外实验) |
DMSO : ~100 mg/mL (~308.27 mM)
|
|---|---|
| 溶解度 (体内实验) |
配方 1 中的溶解度: 2.5 mg/mL (7.71 mM) in 10% DMSO + 90% (20% SBE-β-CD in Saline) (这些助溶剂从左到右依次添加,逐一添加), 悬浮液;超声助溶。
例如,若需制备1 mL的工作液,可将100 μL 25.0 mg/mL澄清DMSO储备液加入900 μL 20% SBE-β-CD生理盐水溶液中,混匀。 *20% SBE-β-CD 生理盐水溶液的制备(4°C,1 周):将 2 g SBE-β-CD 溶解于 10 mL 生理盐水中,得到澄清溶液。 配方 2 中的溶解度: ≥ 2.5 mg/mL (7.71 mM) (饱和度未知) in 10% DMSO + 90% Corn Oil (这些助溶剂从左到右依次添加,逐一添加), 澄清溶液。 例如,若需制备1 mL的工作液,可将 100 μL 25.0 mg/mL 澄清 DMSO 储备液添加到 900 μL 玉米油中并混合均匀。 请根据您的实验动物和给药方式选择适当的溶解配方/方案: 1、请先配制澄清的储备液(如:用DMSO配置50 或 100 mg/mL母液(储备液)); 2、取适量母液,按从左到右的顺序依次添加助溶剂,澄清后再加入下一助溶剂。以 下列配方为例说明 (注意此配方只用于说明,并不一定代表此产品 的实际溶解配方): 10% DMSO → 40% PEG300 → 5% Tween-80 → 45% ddH2O (或 saline); 假设最终工作液的体积为 1 mL, 浓度为5 mg/mL: 取 100 μL 50 mg/mL 的澄清 DMSO 储备液加到 400 μL PEG300 中,混合均匀/澄清;向上述体系中加入50 μL Tween-80,混合均匀/澄清;然后继续加入450 μL ddH2O (或 saline)定容至 1 mL; 3、溶剂前显示的百分比是指该溶剂在最终溶液/工作液中的体积所占比例; 4、 如产品在配制过程中出现沉淀/析出,可通过加热(≤50℃)或超声的方式助溶; 5、为保证最佳实验结果,工作液请现配现用! 6、如不确定怎么将母液配置成体内动物实验的工作液,请查看说明书或联系我们; 7、 以上所有助溶剂都可在 Invivochem.cn网站购买。 |
| 制备储备液 | 1 mg | 5 mg | 10 mg | |
| 1 mM | 3.0827 mL | 15.4135 mL | 30.8271 mL | |
| 5 mM | 0.6165 mL | 3.0827 mL | 6.1654 mL | |
| 10 mM | 0.3083 mL | 1.5414 mL | 3.0827 mL |
1、根据实验需要选择合适的溶剂配制储备液 (母液):对于大多数产品,InvivoChem推荐用DMSO配置母液 (比如:5、10、20mM或者10、20、50 mg/mL浓度),个别水溶性高的产品可直接溶于水。产品在DMSO 、水或其他溶剂中的具体溶解度详见上”溶解度 (体外)”部分;
2、如果您找不到您想要的溶解度信息,或者很难将产品溶解在溶液中,请联系我们;
3、建议使用下列计算器进行相关计算(摩尔浓度计算器、稀释计算器、分子量计算器、重组计算器等);
4、母液配好之后,将其分装到常规用量,并储存在-20°C或-80°C,尽量减少反复冻融循环。
计算结果:
工作液浓度: mg/mL;
DMSO母液配制方法: mg 药物溶于 μL DMSO溶液(母液浓度 mg/mL)。如该浓度超过该批次药物DMSO溶解度,请首先与我们联系。
体内配方配制方法:取 μL DMSO母液,加入 μL PEG300,混匀澄清后加入μL Tween 80,混匀澄清后加入 μL ddH2O,混匀澄清。
(1) 请确保溶液澄清之后,再加入下一种溶剂 (助溶剂) 。可利用涡旋、超声或水浴加热等方法助溶;
(2) 一定要按顺序加入溶剂 (助溶剂) 。
Antidepressant Controlled Trial for Negative Symptoms in Schizophrenia
CTID: NCT01032083
Phase: Phase 4 Status: Completed
Date: 2024-03-28